Jun 12 2014
Scientists at EPFL have invented a molecule that can easily and quickly show how much drug is in a patient’s system. The molecule, now the basis of a start-up company, is expected to enable point-of-care therapeutic drug monitoring.
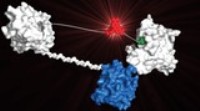 A 3D model of a LUCID molecule © Kai Johnsson/EPFL
A 3D model of a LUCID molecule © Kai Johnsson/EPFL
Monitoring the drug concentration in patients is critical for effective treatment, especially in cases of cancer, heart disease, epilepsy and immunosuppression after organ transplants. However, current methods are expensive, time-consuming, and require dedicated personnel and infrastructure away from the patient. Publishing in Nature Chemical Biology, scientists at EPFL introduce novel light-emitting sensor proteins that can quickly and simply show how much drug is in a patient’s bloodstream by changing the color of their light. The method is so simple that it could be used by patients themselves.
A new molecule enables quick drug dose monitoring
Effective drug treatment relies on balancing the efficiency and toxicity of the drug, which lies at the core of personalized medicine. But as each patient differs from another, this requires constant monitoring in order to best customize drug dosage and prevent side-effects or even poisoning. Current drug-monitoring methods rely on techniques that require specialized personnel and expensive devices, and have to be carried out in diagnostic labs away from the patient’s point-of-care. Developing quick, low-cost methods could improve drug therapy at the patient’s bedside or home, especially in areas with poor medical infrastructure.
A new molecule for monitoring drug concentration
Kai Johnsson’s team at EPFL has developed a novel biosensor molecule that can quickly and accurately measure drug concentration in a patient’s system without requiring anything more complicated than a regular digital camera. The molecule is the result of innovative protein engineering and organic chemistry, and has been shown to work on a range of common drugs for cancer, epilepsy and immunosuppression.
The sensor molecule works by binding the drug circulating in the patient’s bloodstream and changing color accordingly. The molecule itself is made up of four components. One component is a receptor protein, which can bind the molecules of the target drug. The second component is a small molecule similar to the target drug, which can bind the drug receptor. The third component is a light-producing enzyme called luciferase, and the fourth is a fluorophore molecule that can modify the color of the luciferase’s light when it comes close to it.
When there is no drug around, the receptor and the drug-like molecule bind together. This brings the fluorophore close to the luciferase enzyme, and the system produces a red light. But in the presence of drug, e.g. in the blood of a patient, the drug molecules bind the receptor more efficiently and therefore “push” the drug-like molecule off it. The whole sensor molecule system opens up, taking the fluorophore away from the luciferase. As a result, the emitted light turns gradually from red to blue in proportion to the concentration of the drug.
The doctor or the patient can record the signal very easily by putting a drop of sample, e.g. blood, onto a piece of paper, placing it in a dark box and photographing it with a conventional camera. The photograph can then be analyzed by color-measuring software to generate an average measurement. By comparing this measurement to a standard drug-concentration curve, it is easy to calculate the drug concentration in a sample or a patient’s bloodstream. The sensor molecule can be used with virtually any kind of drug, as it simply requires changing the receptor protein on one end and the drug-like molecule on the other.
Successfully tested against anti-cancer and other drugs
The EPFL scientists have called their new class of biosensors “LUCiferase-based Indicators of Drugs”, or LUCIDs. To test their versatility, they developed LUCIDs against six commercially available drugs, including three immunosuppressants, one anti-epileptic, one anti-arrhythmic, and one anti-cancer drug. The drugs were successfully tested in vitro, and the anti-cancer one was also tested against actual human blood-plasma samples. The signal from all six LUCIDs was shown to be accurate and very stable, lasting for more than 10 minutes.
“This system is a cheap, effective solution for customizing drug dosage in patients across a whole array of diseases”, says Rudolf Griss, one of the authors. The successful achievement has encouraged him and co-author Alberto Schena to develop a start-up company in order to streamline and commercialize the innovation. “We envision a simple, hand-held detector where the patient can take a pin-prick of blood and can have an immediate reading of free drug concentration in their system – much like diabetics do now for blood glucose.”