Aug 30 2014
Almost a hundred years ago in 1929 Linus Pauling presented the famous Pauling’s Rules to describe the principles governing the structure of complex ionic crystals. These rules essentially describe how the arrangement of atoms in a crystal is critically dependent on the size of the atoms, their charge and type of bonding.
According to scientists from the Biohybrid Materials Group of Aalto University Finland led by Mauri Kostiainen similar rules can be applied to prepare ionic colloidal crystals consisting of oppositely charged proteins and virus particles. The results can be applied for example in packing and protecting virus particles into crystals that mimic Nature’s own occlusion bodies (protein lattices that pack and protect virus particles to maintain their long-term infectivity), preparation of biocompatible metamaterials, biomolecule crystallization and the subsequent structural analysis.
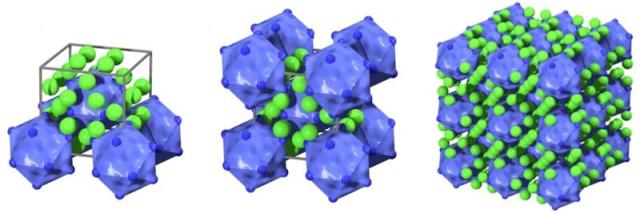 Plug n’ Play Protein Crystals: Virus-avidin nanoparticle crystal structures
Plug n’ Play Protein Crystals: Virus-avidin nanoparticle crystal structures
Viruses, which are commonly perceived only as unwanted infectious agents delivering diseases, can be used also to our benefit. Evolution has rendered virus particles with a precisely defined monodisperse structure, which can be utilized for example as template for nanoparticle synthesis and assembly or as a vehicle to deliver drugs or other active ingredients to living organisms. For example in a previous work from the same research group published in Nano Letters they were able to transfect human cells efficiently with DNA origami nanostructures encapsulated inside virus particles.
In the present work (Ville Liljeström et al. Self-Assembly and Modular Functionalization of Three Dimensional Crystals from Oppositely Charged Proteins, Nature Communications, 2014, 5, 4445) Kostiainen and his research team show that cowpea chlorotic mottle virus (CCMV) particles and avidin proteins can form crystals simply by mixing the two components at an optimized electrolyte concentration. The two components are able to self-assemble into ordered structures due to the charge complementarity presented on their surface. Using avidin as a structural component offers several advantages. Most importantly, avidin is able to bind water-soluble B7-vitamin, biotin, with very high affinity and selectivity. “This enables us to functionalize the crystals in a modular way with almost any biotin tagged ligand. We have demonstrated that it is possible to load the crystals with for example fluorescent dyes, active enzymes and plasmonic gold nanoparticles. Ultimately, using the avidin-biotin interaction allows us to avoid tedious covalent modification of the structures and mimic the process of topotactic intercalation (the insertion of a new component to lattice points of an existing crystal),” Kostiainen says.
The current work deals with only one type of virus particle. “In the future, we will be looking into other virus particles and proteins to ‘glue’ them together”, he adds. “ Studying the assembly of for example human viruses or viruses with other structural topology, such as rod-like particles, may open further possibilities for biomedical and materials science related research.