Sep 10 2014
Defects damage the ideal properties of many two-dimensional materials, like carbon-based graphene. Phosphorus just shrugs.
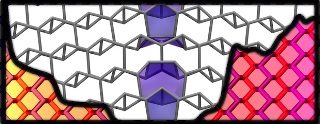 Grain boundaries are rows of defects that disrupt the electronic properties of two-dimensional materials like graphene, but new theory by scientists at Rice University shows no such effects in atomically flat phosphorus. That may make the material ideal for nano-electronic applications. Illustration by Evgeni Penev
Grain boundaries are rows of defects that disrupt the electronic properties of two-dimensional materials like graphene, but new theory by scientists at Rice University shows no such effects in atomically flat phosphorus. That may make the material ideal for nano-electronic applications. Illustration by Evgeni Penev
That makes it a promising candidate for nano-electronic applications that require stable properties, according to new research by Rice University theoretical physicist Boris Yakobson and his colleagues.
In a paper in the American Chemical Society journal Nano Letters, the Rice team analyzed the properties of elemental bonds between semiconducting phosphorus atoms in 2-D sheets. Two-dimensional phosphorus is not theoretical; it was recently created through exfoliation from black phosphorus.
The researchers compared their findings to 2-D metal dichalcogenides like molybdenum disulfide; these metal compounds have also been considered for electronics because of their inherent semiconducting properties. In pristine dichalcogenides, atoms of the two elements alternate in lockstep. But wherever two atoms of the same element bond, they create a point defect. Think of it as a temporary disturbance in the force that could slow electrons down, Yakobson said.
Semiconductors are the basic element of modern electronics that direct and control how electrons move through a circuit. But when a disturbance deepens a band gap, the semiconductor is less stable. When chaos reigns in the form of multiple point defects or grain boundaries — where sheets of a 2-D material merge at angles, forcing like atoms to bond – the materials become far less useful.
The Yakobson lab’s calculations show phosphorus has no such problem. Even when point defects or grain boundaries exist, the material’s semiconducting properties are stable. Like perfect graphene – but unlike imperfect graphene — it performs as expected.
View 2-D phosphorus from above and it looks like graphene, boron nitride or other dichalcogenides, with its rows of hexagons. But at an angle, phosphorus reveals its true form, as alternate atoms jut out of the matrix. This complexity gives rise to more variations among the defects, Yakobson said.
“Because 2-D phosphorus has only one type of element, its defects do not contain hetero-elemental ‘wrong’ bonds,” said Yuanyue Liu, the paper’s first author and a Rice alumnus, now a postdoctoral researcher at the National Renewable Energy Laboratory. “These bonds would not trap or recombine electrons or holes.
“This is a good property for application in solar cells,” he said. “Two-D phosphorus could potentially be used to harvest sunlight, as its band gap matches well with the solar spectrum.” Unlike conventional absorbers, he said, the presence of defects would not deteriorate the material’s performance.
The researchers also show it may be possible to tune the electronic properties of 2-D phosphorus by altering (aka doping) it with foreign atoms. This should be of value to electronics manufacturers, Yakobson said. Carbon and zinc may boost positive conductivity, while potassium may increase negative conductivity; the researchers believe phosphorus may be a promising anode material for batteries.
In fact, 2-D phosphorus has more in common with three-dimensional silicon, the most common element in semiconducting electronics like computer chips. As in 2-D phosphorus, grain boundaries in silicon don’t cause band-gap changes. However, point defects in silicon can change its properties, unlike point defects in phosphorus.
This suggests 2-D phosphorus could also be a candidate for high-performance electronics. In fact, Liu said, several experimental reports have already shown it can be a better transistor than 2-D metal dichalcogenides.
The researchers noted that phosphorus is abundant and black phosphorus can be made relatively easily, but phosphorus reacts slowly with oxygen. To make it practical for daily applications, it has to be well-sealed, Liu said.
The paper’s co-authors include graduate students Fangbo Xu and Ziang Zhang and research scientist Evgeni Penev, all of Rice. Yakobson is Rice’s Karl F. Hasselmann Professor of Materials Science and NanoEngineering, a professor of chemistry and a member of Rice’s Richard E. Smalley Institute for Nanoscale Science and Technology.
The Department of Energy supported the research. The researchers used the Data Analysis and Visualization Cyberinfrastructure supercomputer supported by the National Science Foundation (NSF) and administered by Rice’s Ken Kennedy Institute for Information Technology, as well as NSF’s XSEDE and the Department of Energy’s National Energy Research Scientific Computing Center supercomputers.