May 15 2018
The electrochemical performance of lithium ion batteries (LIBs) has been demonstrated by scientists from the Toyohashi University of Technology with the help of phosphorus-encapsulated carbon nanotube electrodes. In these electrodes, red phosphorus with much higher capacity is inserted into the inner spacing of carbon nanotubes (CNTs) that have a tubular structure.
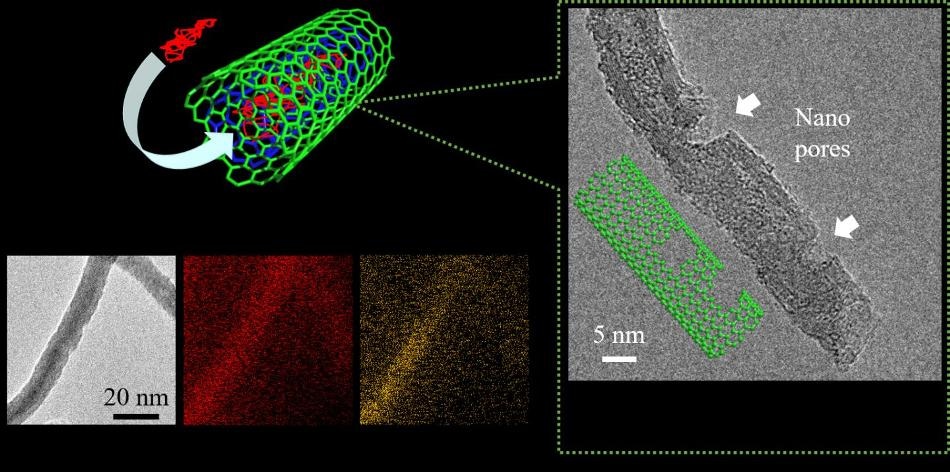 (Left) Elemental mapping images for phosphorus-encapsulated carbon nanotubes with nanopores on the sidewalls. (Right) Transmission electron image of carbon nanotube with nanopores on the sidewalls. (Image credit: Toyohashi University of Technology)
(Left) Elemental mapping images for phosphorus-encapsulated carbon nanotubes with nanopores on the sidewalls. (Right) Transmission electron image of carbon nanotube with nanopores on the sidewalls. (Image credit: Toyohashi University of Technology)
The electrodes exhibited an increase in the electrochemical reactivity of red phosphorus upon formation of accessible lithium ion pathways (or nanopores) onto the sidewalls of the CNTs into which the red phosphorus was introduced. In addition, the structural analysis and charge-discharge profiles indicated reversible electrochemical reactions and the comparatively higher structural stability of red phosphorus in the nanotubes even at the end of the 50th charge-discharge cycle. The charge-discharge capacities are two times or greater than that of graphite used in commercial LIBs. Hence, a proposal for an innovative electrode material for LIBs with higher capacity is made.
Due to the ability of red phosphorus to deliver a theoretical capacity that is nearly seven times greater when compared to the use of graphite as a commercial electrode material for LIBs, it has gained considerable attention as a higher capacitive electrode material for LIBs. The huge variation in the capacity is considered to be due to the presence of admissible amounts of lithium ions in the structures of graphite for LiC6 or phosphorus for Li3P.
However, at the time of the lithium ion insertion and extraction processes, red phosphorus undergoes huge pulverization, volumetric changes, and peeling off, leading to rapid decrease in capacity due to the fall in the amount of electrochemically reactive red phosphorus. Moreover, although electrons travel to the electrode at the time of the lithium ion insertion/extraction, red phosphorus has a drawback in terms of energy loss due to its low electronic conductivity.
Tomohiro Tojo and his team from the Department of Electrical and Electronic Information Engineering, Toyohashi University of Technology, have developed distinctive structures in which red phosphorus is introduced into the inner spacing of CNTs to prevent it from being peeled off from the electrode and to enhance its electronic conductivity. Nanopores, less than 5 nm, were also formed on the sidewalls of the phosphorus-encapsulated CNTs for enhancing the electrochemical reactivity of red phosphorus with the help of the accessible pathways of lithium ions. Following phosphorus encapsulation, the phosphorus atoms were distributed inside the nanotubes, validating the structural stability of red phosphorus.
The reversible capacity demonstrated with the phosphorus-encapsulated CNT electrodes was about 850 mAh/g at the 50th charge-discharge cycle. This value is at least two times greater than that of graphite electrodes. The estimated ratio of charge-discharge capacities, or Coulombic efficiencies, was greater than 99% at the end of the 10th cycle and the subsequent cycles, revealing a high reversibility of charge-discharge reactions on red phosphorus. However, the charge-discharge capacities started to slowly decrease with increase in the number of cycles due to the dissociation of certain P-P bonds and other side reactions on the surface of phosphorus and the CNTs.
Fascinatingly, the phosphorus-encapsulated CNT that included nanopores led to considerable increase in electrochemical performance than the phosphorus-encapsulated CNT without any nanopores. This is proposed to be because of the high reactivity of red phosphorus with lithium ions with the help of the nanopores on the sidewalls. At the end of the charge-discharge cycles, it was observed that red phosphorus was inside the nanotubes.
The researchers propose the phosphorus-encapsulated CNTs as an electrode material for LIBs with higher capacity, despite the fact that further advancements in the structures are needed to accomplish long-term cycling without any dip in capacity. More research will be carried out related to the utilization of such electrodes.
Financial support through the Murata Science Foundation, Public Interest Tatematsu Foundation, and the Public Foundation of the Chubu Science and Technology Center is acknowledged by Tomohiro Tojo.