Jun 12 2015
Small heat shock proteins ensure that other proteins do not clot, allowing the cell to survive stress. Defects in these "small helpers" are associated with medical conditions like cataracts and cancer. Now, scientists at the Technische Universität München (TUM) have characterized a small heat shock protein responsible for embryonic development in the Caenorhabditis elegans nematode. Presumably, a similar protein exists also in humans.
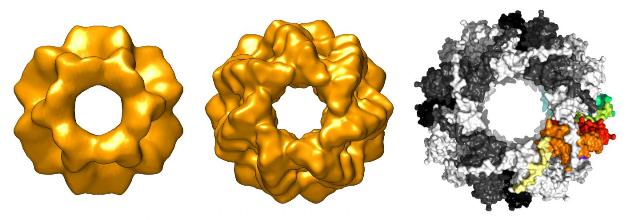 The small heatshock protein Spi1 exists in three forms comprising 24, 28 and 32 identical subunits. Acidic conditions shift the equilibrium towards the active forms with 24 and 28 subunits. Credit: Image: Tilly Fleckenstein / Sevil Weinkauf / TUM
The small heatshock protein Spi1 exists in three forms comprising 24, 28 and 32 identical subunits. Acidic conditions shift the equilibrium towards the active forms with 24 and 28 subunits. Credit: Image: Tilly Fleckenstein / Sevil Weinkauf / TUM
Like humans, cells often face catastrophic situations. Even though cells are not threatened by hurricanes and earthquakes, the damage induced by heat or radiation is equally devastating: Important proteins that control chemical reactions, transport substances or recognize signal substances, among others, lose their structure and are rendered useless. When this happens, cell processes go out of control.
But cells have their own catastrophe prevention systems: Small heat shock proteins prevent other proteins from clotting into irregular clumps to ensure that they can retain their proper structure. This allows them to continue performing their tasks - the cells survive. Nine of these helper proteins are currently known in the human body, and they are very versatile. They do their work in all kinds of tissue: in the brain, in heart and muscles, as well as in the lenses of our eyes where they prevent turbidity.
When small heat shock proteins cease to function properly, a broad range of ailments like cataracts, certain neuronal conditions or cancer can ensue. Scientists are thus interested in understanding precisely what the various heat shock proteins are responsible for, what their molecular structures look like and how they are regulated.
A protective protein specifically for embryonic development
A group of scientists at the TU München led by Johannes Buchner, Sevil Weinkauf, and Michael Groll, have now for the first time successfully characterized the molecular structure and function of a small heat shock protein that exists exclusively in the eggs and embryos of the Caenorhabditis elegans nematode.
The researchers determined that the Sip1 protein is responsible specifically for the development of embryos and that it is regulated via the pH value rather than temperature. "Sip1 is the only small heat shock protein that we are aware of exhibiting these properties," says Buchner. "It assumes the difficult task of maintaining the protein balance in a quickly dividing tissue and an acidic environment during the embryonic phase. No other heat shock protein can do the same." The members of the small heat shock protein family effectively share the work - with some specialization for preventing different catastrophe scenarios.
Even though Sip1 exists only in nematodes, the implications for humans are interesting. "We do not yet know whether a protein with a similar function also plays a role in human embryonic development, but we suspect so," says Buchner. The great similarity between the small heat shock proteins of nematodes and humans in specific locations becomes apparent in the crystal structure of the Sip1 protein. The manner in which two key parts of the protein complex interact is remarkably similar to that of the alpha-B-crystallin in the lens of the human eye.
Prefabrication principles in protective proteins
Understanding the connection between structure, regulation and function of the Sip1 proteins was possible only as a result of the close collaboration between scientists from different research areas at the Department of Chemistry. In biological and biochemical experiments they first determined the significance of Sip1 for the survival of nematode embryos. They discovered how the heat shock protein prevents clotting of important embryonic proteins during heat stress when activated by a low pH value.
Electron microscopic and crystal structure analyses put a further piece of the puzzle into place. They showed that the protein is present in multiple forms comprising either 32, 28 or 24 identical subunits. In high pH environments, large complexes dominate and the protein is inactive. When, however, the pH value becomes acidic, the large oligomers dissociate and the protective protein is activated.
In future projects the scientists led by Buchner, Weinkauf and Groll hope to identify the molecular switch that triggers the dissociation of large Sip1 oligomers into the smaller ones.