Researchers from Georgia Tech, University of Wisconsin-Madison, Oak Ridge National Laboratory, Arizona State University and Xiamen University in China have developed a new fabrication method that minimizes the need for expensive metal to induce catalytic activity in fuel cell applications. The new method allows the production of hollow platinum nanocages with ultra-thin walls.
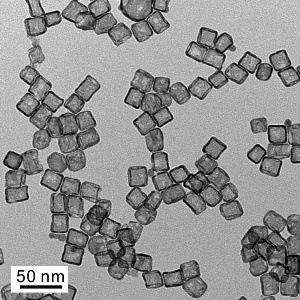 A transmission electron microscope image shows a typical sample of platinum cubic nanocages. (Credit: Xia Laboratory)
A transmission electron microscope image shows a typical sample of platinum cubic nanocages. (Credit: Xia Laboratory)
These atomic-scale layers of platinum are produced through a solution-based approach so as to form hollow, porous structures that induce catalytic activity within and outside the nanocages. The layers are grown on templates of palladium nanocrystal templates. The palladium is etched off, leaving behind nanocages with a diameter of approximately 20 nm, and between three and six atom-thin platinum layers.
When these nanocage structures are used in fuel cell electrodes, platinum's utilization efficiency can be increased by a factor of seven, which could affect the economic viability of the fuel cells.
“We can get the catalytic activity we need by using only a small fraction of the platinum that had been required before,” said Younan Xia, a professor in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory University. Xia also holds joint faculty appointments in the School of Chemistry and Biochemistry and the School of Chemical and Biomolecular Engineering at Georgia Tech. “We have made hollow nanocages of platinum with walls as thin as a few atomic layers because we don’t want to waste any material in the bulk that does not contribute to the catalytic activity.”
Platinum as a catalyst holds significant importance in different industrial and consumer applications. However, due to its high cost, the use of low-temperature fuel cells in automobile and home applications has been limited.
The chemical reactions involved in the catalytic applications are supported by the platinum surface layers, which have enabled the researchers to produce new structures that increase the platinum's exposure to reactants. The amount of precious metal that does not support the reaction is reduced through the hollowing out process. This process also enables the use of larger nanocrystals that are less likely to be harmed by sintering - an aggregation process in which the catalyst surface area is reduced.
“We can control the process so well that we have layer-by-layer deposition, creating one layer, two layers or three layers of platinum,” said Xia, who is also a Georgia Research Alliance eminent scholar. “We can also control the arrangement of atoms on the surface so their catalytic activity can be engineered to fit different types of reactions.”
He further stated that hollow platinum structures have been produced previously, but their walls were not as thin as these. Earlier examples had wall thicknesses of approximately 5 nm. This new method is capable of producing shell walls with a thickness of less than 1 nm. As both the inner and outer layers of the porous nanocages play a vital role in catalytic activity, it is possible for the new structures to use a maximum of two-thirds of the platinum atoms in an ultra-thin three-layer shell. However, traces of palladium are found to be present with the platinum in the structures.
Xia commented:
This approach creates the highest possible surface area from a given amount of platinum.
With the use of palladium nanocrystals as templates, the nanocages can be formed in either cubic or octahedral shapes. The surface structure is controlled by the shape of the nanocages, which further leads to modifications in the catalytic activity.
The research is mainly focused on reducing the expense of cathodes used in fuel cells that power homes and automobiles. The oxygen-reduction reaction occurring at the cathode in the fuel cell requires platinum in substantial quantities. The hollow shells could result in economically beneficial automotive and home fuel cells by minimizing the amount of platinum by up to a factor of seven.
Upon evaluating the durability of the platinum nanocages for oxygen-reduction reaction, the researchers observed that there is a fall in the catalytic activity by slightly more than one-third after 10,000 operating cycles. Previous work carried out in increasing surface area was based on tiny platinum nanoparticles 2 or 3 nm in diameter. These particles showed a tendency to clump together via the sintering process, thereby minimizing the surface area.
“By using hollow structures, we can use much larger particle sizes – about 20 nanometers – and we really don’t lose any surface area because we can use both the inside and outside of the structure, and the shells are only a few atomic layers thick,” Xia commented. “We expect the durability of these larger particles to be much better.”
Other applications, including catalytic converters in automobiles, employ significant quantities of platinum. The application of new hollow shells in automobile catalytic converters is limited as these converters function at temperatures that cannot be tolerated by the shells. However, the platinum nanocages could be used in other industrial processes such as hydrogenation.
Besides carrying out experimental work at Georgia Tech, researchers at Arizona State University and Oak Ridge National Laboratory mapped the nanocage structures using their specialized microscopy facilities. Researchers at the University of Wisconsin-Madison designed the system such that the etching of palladium from the core could be understood, and the platinum shell could be preserved.
Xia said that although several works have been carried out to explore platinum alternatives, none of the alternatives has yielded the equivalent amount of catalytic activity in an equally small mass until now.
“If you took all of the platinum that we have available today and made a cube, it would only be seven meters on each side,” he added. “That’s all the platinum we have now, so we need to find the most efficient way to use it.”
The co-authors of the paper include Professor Manos Mavrikakis and researchers Luke Roling and Jeffrey Herron from the University of Wisconsin-Madison, Miaofang Chi from Oak Ridge National Laboratory, Professor Jingyue Liu from Arizona State University, Professor Zhaoxiong Xie from Xiamen University, and Lei Zhang, Xue Wang, Sang-Il Choi, Madeleine Vara and Jinho Park, from Georgia Tech.
The findings of the research were published in the July 24 issue of the journal Science.