Nov 5 2015
Inspired by the unique, but extremely ordinary design, of a household gadget a new type of biological marker has been developed. 'Nanotags' are capable of accommodating an extremely high number of fluorophores, with no-self quenching effects, meaning proteins of minute concentrations can be easily visualized in living tissue.
Heiti Paves | Shutterstock
Bottle brushes are one of those everyday items that you rarely even notice. They're used universally in kitchens, where their long stalk with a bristled head are used to clean vases and anything else with a bottleneck.
Fluorescent probes are also used universally, but for tasks that are remarkably more complex. Probes are used to selectively attach to specific proteins and cellular structures to allow for their identification with a microscope. A common use of these probes is the differentiation between cancerous and healthy cells.
A standard method of producing a fluroescent probe is by 'sandwich formation' where a primary antibody, which will selectively bind to a particular cellular protein, is attached to a fluorophore containing secondary antibody, which allows the binding to be observed via a microscope.
However if the protein of interested is at a low cellular concentration this process becomes difficult as the flurophore signal can be too weak to observe. In such situations, a secondary antibody with thousands of fluorophores can be used in order to increase the signal. However, this approach can result in the self-quenching of the flurophores that are in close spatial proximity; resulting in a reduction of the signal intensity.
Taking inspiration from bottle brushes, which that features many bristles, scientists Subha R. Das and Bruce Armitage working in the labs of Krzysztof Matyjaszewski wored to resolve this problem by developing brush-shaped polymers which include side chains, which are similar to bristles.
The ends of these side chains were connected by DNA and then complementary DNA was used to give a double strand. The researchers then added unique fluorescent molecules which adhered within the double-stranded DNA molecules.
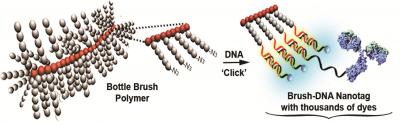 A bottle-brush polymer is modified with DNA, loaded with thousands of dye molecules and attached to an antibody to visualize cell-surface targets. Credit:American Chemical Society
A bottle-brush polymer is modified with DNA, loaded with thousands of dye molecules and attached to an antibody to visualize cell-surface targets. Credit:American Chemical Society
This special bottle-brush structure acts as a new secondary antibody which can bind to an almost-infinitely large number of fluorophores with no quenching, resulting in significant signal amplification.
According to the team the brushes can be adjusted to deliver cancer therapeutics.