Jan 13 2016
A team of researchers from Stanford University have constructed the first lithium-ion battery capable of turning on and off according to the temperature.
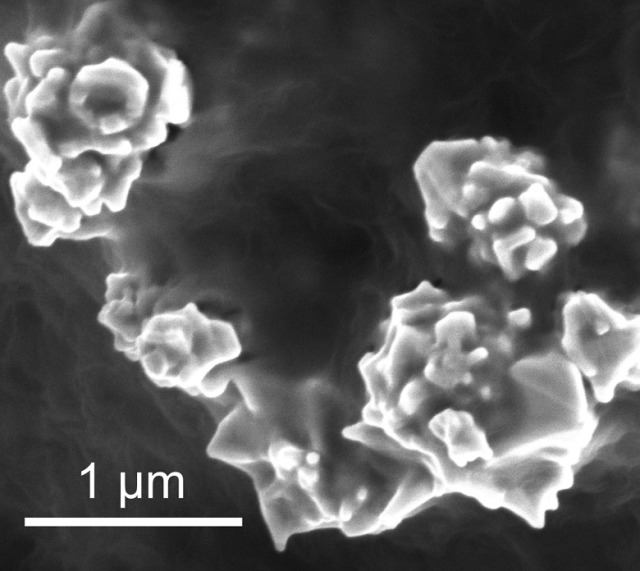 Bunched together, as shown here, nanoparticles of graphene-coated nickel conduct electricity. When the battery overheats, the particles separate and electric current stops flowing. During cooling, the particles reunite and the battery starts producing electricity again. (Photo credit: Zheng Chen)
Bunched together, as shown here, nanoparticles of graphene-coated nickel conduct electricity. When the battery overheats, the particles separate and electric current stops flowing. During cooling, the particles reunite and the battery starts producing electricity again. (Photo credit: Zheng Chen)
The battery shuts off before overheating and restarts when the temperature has cooled. This technology could prevent battery fires that have affected several battery-operated products such as laptops, computers, navigation systems, recliners, hoverboards, and electronic devices, and caused them to be re-called or banned.
People have tried different strategies to solve the problem of accidental fires in lithium-ion batteries. We've designed the first battery that can be shut down and revived over repeated heating and cooling cycles without compromising performance.
Zhenan Bao, Professor of Chemical Engineering at Stanford.
The new battery was introduced by Bao and her Stanford colleagues in the January 11th issue of the Nature Energy journal.
A standard lithium-ion battery has two electrodes and a gel or liquid electrolyte which transports charged particles between the electrodes. The battery generates heat when it is punctured, overcharged or shorted, and if the temperature reaches around 300 degrees Fahrenheit (150 degrees Celsius), the electrolyte could catch fire and trigger an explosion.
Many techniques have been tested to prevent battery fires, for example, flame retardants were added to the electrolyte. In another case, a "smart" battery was built by a Stanford engineer Yi Cui, which was capable of providing early warning prior to the battery becoming too hot.
Unfortunately, these techniques are irreversible, so the battery is no longer functional after it overheats. Clearly, in spite of the many efforts made thus far, battery safety remains an important concern and requires a new approach.
Yi Cui, Associate Professor of Materials Science and Engineering and of Photon Science.
Cui, Bao and postdoctoral scholar Zheng Chen focused on nanotechnology to find a solution for the issue. Recently, Bao had built a wearable sensor capable of monitoring human body temperature. The sensor is made of plastic material embedded with minuscule nickel particles with nanoscale spikes on its surface.
For the battery research, the team coated graphene on the spiky nickel particles and embedded the particles into a thin film of elastic polyethylene.
We attached the polyethylene film to one of the battery electrodes so that an electric current could flow through it. To conduct electricity, the spiky particles have to physically touch one another. But during thermal expansion, polyethylene stretches. That causes the particles to spread apart, making the film nonconductive so that electricity can no longer flow through the battery.
Zheng Chen, Lead Author, Postdoctorial Scholar.
They observed the polyethylene film start to inflate like a balloon when the battery was heated at more than 160°F (70°C), causing the spiky particles to separate and the battery to switch off. When the temperature returned to 70°C, the polyethylene film deflated and the particles returned to their former state, enabling the battery to start generating electricity again.
We can even tune the temperature higher or lower depending on how many particles we put in or what type of polymer materials we choose. For example, we might want the battery to shut down at 50°C or 100°C.
Zhenan Bao, Professor of Chemical Engineering at Stanford.
Safe & reliable lithium-ion battery
The Stanford team continued to apply heat on the new material with a hot-air gun to test the material’s stability. The battery repeatedly shut down when it got too hot and swiftly restarted once the temperature cooled.
Compared with previous approaches, our design provides a reliable, fast, reversible strategy that can achieve both high battery performance and improved safety. This strategy holds great promise for practical battery applications.
Yi Cui, Associate Professor of Materials Science and Engineering and of Photon Science.
Stanford postdoctoral scholars Nan Liu, Chao Wang, Sean Andrews and Jia Liu; and graduate students Po-Chun Hsu, Jeffrey Lopez, Yuzhang Li and John To were also involved in this research.
The SLAC National Accelerator Laboratory and the Precourt Institute for Energy at Stanford supported this research.