Sep 12 2016
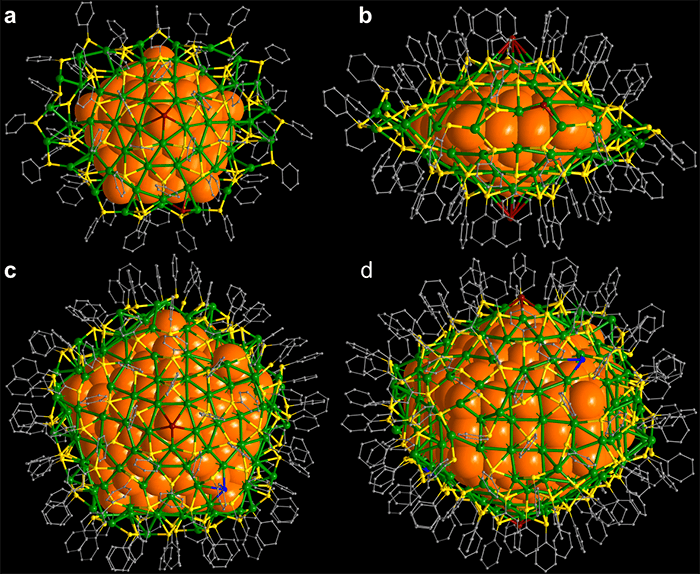 This figure shows: Upper row: (a) top and (b) side view of the 136-atom silver nanocluster. Lower row: (c) top and (d) side view of the 374-atom silver nanocluster. The metal cores of these clusters have a diameter of 2 and 3 nm, respectively. Silver atoms in the metal core are denoted by large orange sphere. The core is protected by a silver-thiol layer (green: silver; yellow: sulfur; carbon: gray). Courtesy of Nanfeng Zheng, Xiamen University. (Credit: University of Jyväskylä)
This figure shows: Upper row: (a) top and (b) side view of the 136-atom silver nanocluster. Lower row: (c) top and (d) side view of the 374-atom silver nanocluster. The metal cores of these clusters have a diameter of 2 and 3 nm, respectively. Silver atoms in the metal core are denoted by large orange sphere. The core is protected by a silver-thiol layer (green: silver; yellow: sulfur; carbon: gray). Courtesy of Nanfeng Zheng, Xiamen University. (Credit: University of Jyväskylä)
A group of international scientists have successfully synthesized and characterized two previously unknown, record-large silver nanoclusters of 136 and 374 atoms.
Scientists from China, Finland, Germany and Australia have synthesized these diamond-shaped nanoclusters that comprise of a protective layer of organic thiol molecules and silver atoms surrounding a silver core of two or three nanometers. These nanoclusters are the largest ones whose structure is now known to atomic precision.
The details of the research have been published on 9th September 2016, in the journal Nature Communications.
The synthesis of the nanoclusters was done at Xiamen University, China, while the characterization process performed through electron microscopy and X-ray crytallography happened in China, Germany and Australia. The optical properties and electronic structure of the nanoclusters was then computationally studied in Finland at the Nanoscience Center (NSC) of the University of Jyväskylä.
The existence of gold nanoclusters stabilized by a thiol molecular layer has been known for many years, however silver nanoclusters have only attracted the interest of researchers in recent years. As it is cheaper and has better controllable optical properties that are suitable for applications, silver is better suited for nanocluster synthesis. But, synthesis processes that can produce silver clusters are not as well known as those for gold.
"These largest atomically precise silver nanoclusters known thus far serve as excellent model systems to understand how silver nanoparticles grow," says Professor Nanfeng Zheng whose research group prepared the clusters in Xiamen University in China. "The internal structure of the metal core is a combination of little crystallites of silver that are joined together to form a five-fold symmetric diamond-shape structure."
From a theoretical point of view these new clusters are very interesting. These clusters are already big enough that they have properties similar to silver metal, such as strong absorption of light leading to collective oscillations of the electron cloud known as plasmons, yet small enough that we can study their electronic structure in detail. Much to our surprise, the calculations showed that electrons in the organic molecular layer take part actively in the collective oscillation of the silver electrons. It seems possible to then activate these clusters by light in order to do chemistry at the ligand surface.
Hannu Häkkinen, Academy Professor, NSC