Oct 7 2016
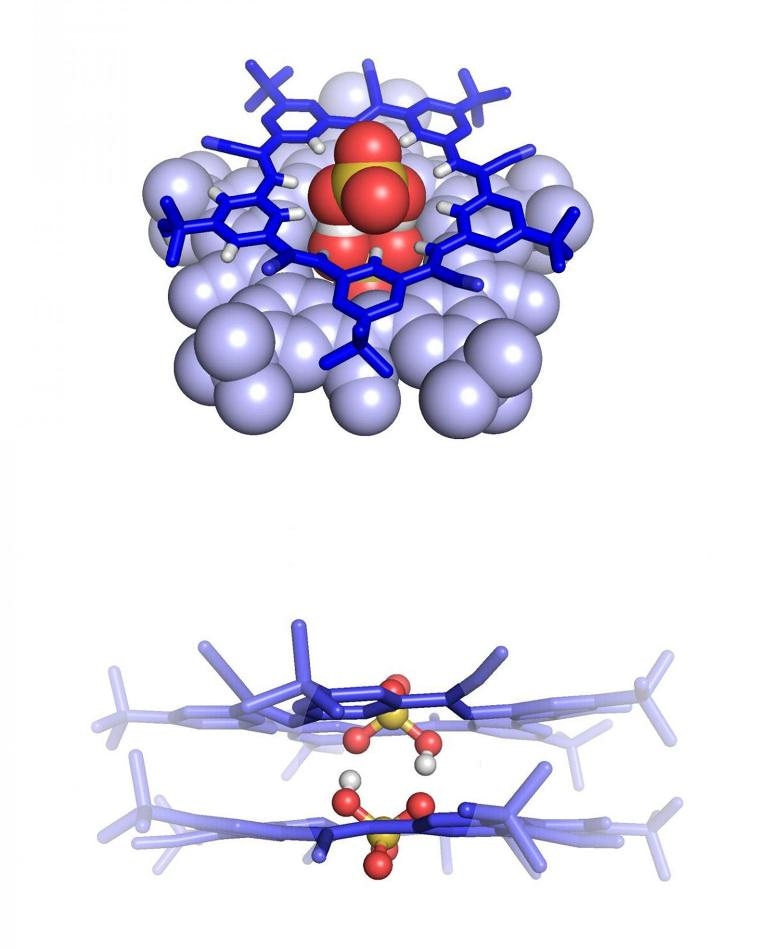 The bisulfate dimer as seen from the top down (upper image) and side (lower image). The two negatively charged molecules are connected by weak bonds made possible through encapsulation inside a pair of a star-shaped cyanostar macrocycles (blue), a molecule previously developed by Flood's lab at IU. (CREDIT: Indiana University)
The bisulfate dimer as seen from the top down (upper image) and side (lower image). The two negatively charged molecules are connected by weak bonds made possible through encapsulation inside a pair of a star-shaped cyanostar macrocycles (blue), a molecule previously developed by Flood's lab at IU. (CREDIT: Indiana University)
The first evidence of new molecular structure has potential for chemical solutions for various environmental issues.
Researchers at Indiana University have detected proof of the existence of a new molecular structure that has properties that could potentially reduce chemicals that pollute water and cause destruction of large fish kills, and also help to store of nuclear waste.
This study provides experimental evidence for the existence of a chemical bond between two negatively charged molecules of bisulfate (HSO4). The results have been published online in the German scientific journal Angewandte Chemie International Edition.
The existence of a supramolecule with two negatively charged ions was once considered to be impossible as it opposes an almost two century old chemical law. This chemical law has come under new scrutiny.
An anion-anion dimerization of bisulfate goes against simple expectations of Coulomb's law, but the structural evidence we present in this paper shows two hydroxy anions can in fact be chemically bonded. We believe the long-range repulsions between these anions are offset by short-range attractions.
Amar Flood, Professor, Indiana University
Flood is a professor in the IU Bloomington College of Arts and Sciences' Department of Chemistry. Elisabeth Fatila, a postdoctoral researcher in Flood's lab, is the first author of the study.
According to molecular chemistry, if two monomer molecules are connected by a strong covalent bond, then it is known as a dimer. As per supramolecular chemistry, the dimers are connected by several weak non-covalent bonds. The negatively charged particles are known as anions and a chain of polymers form a monomer.
The main idea of Coulomb's law is that two molecules possessing the same charge can cause a repellent force which prevents chemical bonding, for instance when the same ends of two magnets are brought close to each other.
However, recently experts have started to argue that negatively charged molecules with hydrogen atoms, for example bisulfate, comprising sulfur, hydrogen and oxygen, has the capability to form viable chemical bonds.
Although supramolecular chemistry started out as an effort to create new molecular hosts that hold on to complementary molecular guests through non-covalent bonds, the field has recently branched out to explore non-covalent interactions between the guests in order to create new chemical species.
Amar Flood, Professor, Indiana University
A self-complementary and anti-electrostatic hydrogen bond is employed in the negatively charged bisulfate dimer that is considered in this IU study.
Encapsulation inside a pair of cyanostar macrocycles which was already developed by Flood's lab at IU enables the existence of the molecules. Fatila and other researchers tried to bind a single bisulfate molecule inside the cyanostar; but the existence of two negatively charged bisulfate ions was unexpected.
This paper is inspirational because it may launch a new approach to supramolecular ion recognition. I expect this will be the start of something new and important in the field.
Jonathan Sessler, Professor of Chemistry, University of Texas
Considering the ability to develop a negatively charged bisulfate dimmer, the search for chemical solutions for various environmental challenges will also advance. Because of the ion-extraction properties of these molecules, they can be used to remove sulfate ions from the process that is used to convert nuclear waste into storable solids.
This process is known as vitrification and it is affected by these sulfate ions. These molecules can also be used to extract destructive phosphate ions present in the environment.
"The eutrophication of lakes is just one example of the serious threat to the environment caused by the runoff of phosphates from fertilizers," Flood said, highlighting the uncontrolled growth of plants that occurs due to excess phosphate nutrients running into oceans and lakes. When these chemicals enter any water source as runoff from fertilizers that are produced from dairy farms and used to increase crop yields, they tend to provoke massive algae blooms that in turn contaminate water supplies and destroy the marine ecosystem to a large extent.
Flood was recognized as a principal investigator on a new, separate grant from the National Science Foundation that mainly focuses on removing these substances from the environment. This $600,000 grant is a collaboration with Heather Allen, a professor at The Ohio State University, which is also near a country that has recently experienced large algae blooms because of agricultural runoff into Lake Erie. This grant is issued for three years and was awarded in the month of August, 2016.
This new molecule was found using equipment at the IU Bloomington Department of Chemistry's Nuclear Magnetic Resonance Facility, the Laboratory for Biological Mass Spectrometry and the IU Molecular Structure Center. Professor Krishnan Raghavachari, associate scientist Jonathan A. Karty, research associate Eric B. Twum, senior scientist Maren Pink and Ph.D. student Arkajyoti Sengupta, all from the IU Bloomington Department of Chemistry, are co-authors of this study.
Pink is also director of the IU Molecular Structure Center. Karty is the manager for the Mass Spectrometry lab. Twum is a spectroscopist in the Nuclear Magnetic Resonance Facility.
The National Science Foundation supported this research project. This study was presented at the 11th International Symposium on Macrocyclic and Supramolecular Chemistry in Seoul, South Korea, for the first time.
The cynostar macrocycle that was founded by Flood's laboratory is the subject of a pending patent that is filed by the IU Research and Technology Corp.