Sep 20 2017
Researchers from the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) have created an innovative electrocatalyst with the ability to directly convert carbon dioxide into alcohols and multicarbon fuels with the need for very low energy inputs.
The study is the most recent one, among others, from Berkeley Lab that concentrates on overcoming the difficulties in developing a clean chemical manufacturing system with the ability to efficiently use carbon dioxide.
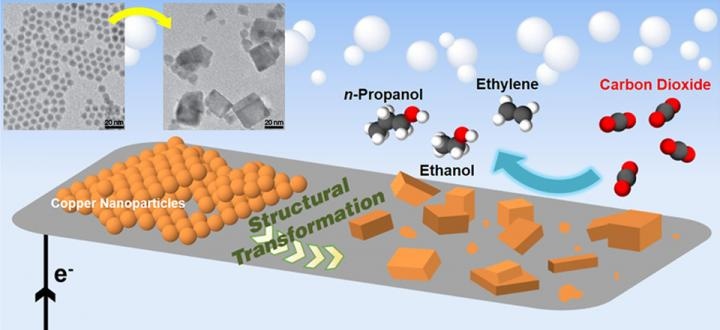 Schematic of a new catalyst made of copper nanoparticles that converts carbon dioxide to multicarbon products (ethylene, ethanol, and propanol). At top left are transmission electron microscope images of the copper nanoparticles. The transformation of the nanoparticles from spheres to cube-like structures is key to keeping the energy input low for the reactions. CREDIT: Dohyung Kim/Berkeley Lab.
Schematic of a new catalyst made of copper nanoparticles that converts carbon dioxide to multicarbon products (ethylene, ethanol, and propanol). At top left are transmission electron microscope images of the copper nanoparticles. The transformation of the nanoparticles from spheres to cube-like structures is key to keeping the energy input low for the reactions. CREDIT: Dohyung Kim/Berkeley Lab.
The study was published in the Proceedings of the National Academy of Sciences this week. In the study, Researchers headed by Peidong Yang, a Berkeley Lab Scientist, found that an electrocatalyst developed by using copper nanoparticles presented the conditions inevitable for disintegrating carbon dioxide to synthesize products such as ethanol, ethylene and propanol.
These products include two or three carbon atoms, and are regarded to be of high value to the current lifestyle. Ethylene is the basic component for manufacturing bottles and plastic films, and also polyvinyl chloride (PVC) pipes. For many years now, ethanol (usually produced from biomass) has been used as a biofuel additive for gasoline. Although propanol is a highly efficient fuel, at present it is highly expensive to be produced to be used as fuel.
In order to calculate the catalyst’s energy efficiency, Researchers take into account the thermodynamic capacity of products (i.e. the energy quantity gained from an electrochemical reaction) as well as the extra voltage required more than the thermodynamic capacity to initiate the reaction at adequate reaction rates. The extra voltage is known as the overpotential, where the catalyst is more efficient when the overpotential is lower.
It is now quite common in this field to make catalysts that can produce multicarbon products from CO2, but those processes typically operate at high overpotentials of 1 volt to attain appreciable amounts. What we are reporting here is much more challenging. We discovered a catalyst for carbon dioxide reduction operating at high current density with a record low overpotential that is about 300 millivolts less than typical electrocatalysts.
Peidong Yang, Senior Faculty Scientist, Berkeley Lab’s Materials Sciences Division
Cube-like copper
The team used transmission electron microscopy, X-ray photoelectron spectroscopy and scanning electron microscopy at Berkeley Lab’s Molecular Foundry to characterize the electrocatalyst.
The catalyst was found to include densely packed copper spheres layered on carbon paper in a tightly packed manner, where the diameter of each copper sphere was nearly 7 nm. The team discovered that through the initial phase of electrolysis, nanoparticle clusters fused together and transformed into cube-shaped nanostructures. The size of the cube-form shapes was 10-40 nm.
It is after this transition that the reactions to form multicarbon products are occurring. We tried to start off with pre-formed nanoscale copper cubes, but that did not yield significant amounts of multicarbon products. It is this real-time structural change from copper nanospheres to the cube-like structures that is facilitating the formation of multicarbon hydrocarbons and oxygenates.
Dohyung Kim, Lead Author of the study and a Graduate Student, Berkeley Lab’s Chemical Sciences Division and Department of Materials Science and Engineering
The precise nature of this transitioning is unpredictable to date, stated Yang, who is a Professor at UC Berkeley’s Department of Materials Science and Engineering as well.
“What we know is that this unique structure provides a beneficial chemical environment for CO2 conversion to multicarbon products,” explained Yang. “The cube-like shapes and associated interface may be providing an ideal meeting place where the carbon dioxide, water, and electrons can come together.”
Many paths in the CO2-to-fuel journey
This recent research illustrates the way carbon dioxide reduction has come to be a highly active area in the research on energy for many years now. Contrary to tapping solar energy to transform carbon dioxide into plant food, artificial photosynthesis involves using the same basic constituents to synthesize chemical precursors that are usually used in synthetic products and also fuels such as ethanol.
The Berkeley Lab research team proposes to work on different facets of the problem at hand, for example, end product of the catalytic reactions. In 2016, for example, they created a hybrid semiconductor-bacteria system for synthesizing acetate from sunlight and CO2. At the start of 2017, another team of Researchers adopted a photocatalyst for near-thorough transformation of carbon dioxide to carbon monoxide. In the recent past, they reported an innovative catalyst for the efficacious synthesis of synthesis gas mixtures, that is, syngas.
Scientists have also endeavored to enhance the energy efficiency in the reduction of carbon dioxide to enable the use of these systems in industries.
In a recent paper published by a team headed by Berkeley Lab Researchers at the Joint Center for Artificial Photosynthesis, basic science concepts were used to demonstrate how optimization of every component of a whole system enables achieving the aim of solar-powered fuel production at stunning energy efficiency rates.
The new PNAS research is centered on only the catalyst’s efficiency than the system as a whole. However, the Scientists indicate that the catalyst can be used along with different sources of renewable energy, such as solar cells.
By utilizing values already established for other components, such as commercial solar cells and electrolyzers, we project electricity-to-product and solar-to-product energy efficiencies up to 24.1 and 4.3 percent for two-to-three carbon products, respectively.
Dohyung Kim, Lead Author of the study and a Graduate Student, Berkeley Lab’s Chemical Sciences Division and Department of Materials Science and Engineering
Kim is of the opinion that if this catalyst is integrated with an electrolyzer which forms a part of a solar fuel system, a material measuring just 10 cm2 can synthesize nearly 1.3 gm ethylene, 0.8 gm ethanol and 0.2 gm propanol every day.
“With continued improvements in individual components of a solar fuel system, those numbers should keep improving over time,” explained Kim.
The study was carried out through Berkeley Lab’s Catalysis Research Program which was funded by DOE’s Office of Science. The Molecular Foundry is a DOE Office of Science User Facility.