Mar 29 2018
Rice University scientists claim that it is easy and cost-effective to fabricate shiny pellets of graphite from functionalized graphene.
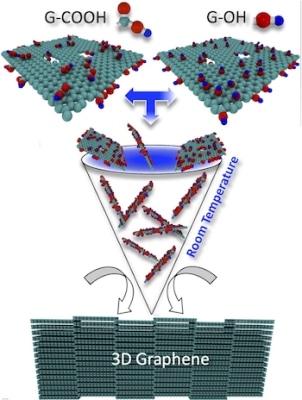 Researchers at Rice University consolidated functionalized graphene at room temperature by first crushing graphene powder and then pressing it into pellets. Chemically altering the graphene powder with carbon, oxygen, and hydrogen provided a way to bind the graphene into a three-dimensional solid under pressure. (Image credit: Ajayan Research Group)
Researchers at Rice University consolidated functionalized graphene at room temperature by first crushing graphene powder and then pressing it into pellets. Chemically altering the graphene powder with carbon, oxygen, and hydrogen provided a way to bind the graphene into a three-dimensional solid under pressure. (Image credit: Ajayan Research Group)
A report published in Carbon demonstrates how graphene powder, which is chemically modified, can be pressed into a semi-porous, lightweight solid that retains most of the powerful and conductive properties of graphite – the form of carbon found in lubricants, pencils, and various other products that usually need high-temperature processing to develop.
A research team, which included Mohamad Kabbani, a former graduate student of Rice materials, and Pulickel Ajayan, scientist and lead author of the paper, showed that the scalable and environmentally friendly process can be done quickly by manually grinding the chemically altered graphene into a powder and then utilizing a hand-powered press to shape the powder into a solid, shiny pellet.
Earlier, Kabbani demonstrated how carbon nanotubes can be converted into graphene by using a mortar and pestle instead of harsh chemicals. Now, this time, he along with his coworkers showed how to make a battery-sized pellet, but the graphene powders that have chemical functionalities attached to it can be easily pressed into any form.
According to Kabbani, the material could be used for catalytic, structural, electronic, and electrochemical applications.
This is the first time anyone’s made these at room temperature and without very high pressure. Usually these kinds of materials require sintering (a process that uses pressure or heat to form solids without melting them) at temperatures above 1,000 degrees Celsius to produce strong pellets. In this case, mechano-chemistry at the nanoscale saved us a lot of energy and money.
Mohamad Kabbani
The procedure starts with two sets of functionalized nanotubes – one with hydroxyl molecules and the other with carboxylic acid. As soon as these nanotubes are crushed to combine them either by machine or hand, they are positioned in a lab-scale hydraulic press and then exposed to 5 tons of pressure. Kabbani informed that the functional groups cross-linked the graphene sheets with one another, and although liquids were not used, they still managed to create a small quantity of water as a byproduct of the reaction. When the pellets were placed in hot water for five hours, they remained stable and continued to do so even when they were stirred. This showed the interlocking of the graphene sheets within, said the researchers.
According to Kabbani, the combination of high conductivity, high strength, and light weight is appealing for applications such as electrodes and conducting cables. “The pellet material is stronger and lighter than commercial graphite electrodes and could be promising for electrical storage applications with high energy and power densities,” he said.
The paper’s co-authors are postdoctoral researchers Vidya Kochat and Matias Soto; Chandra Sekhar Tiwary, a former postdoctoral researcher at Rice and now an assistant professor at IIT Gandhinagar, India; Sanjit Bhowmick and Syed Asif of Bruker Nano Surfaces, Minneapolis; Anirban Som, K.R. Krishnadas, and Thalappil Pradeep of the Indian Institute of Technology, Madras, India; Ahmad Kabbani of the Lebanese American University, Beirut; and Enrique Barrera, a professor of materials science and nanoengineering, and Robert Vajtai, an associate research professor of materials science and nanoengineering, both at Rice University.
Ajayan is chair of Rice’s Department of Materials Science and NanoEngineering, the Benjamin M. and Mary Greenwood Anderson Professor in Engineering and a professor of chemistry. Mohamad Kabbani is now a research scientist at Shell International Exploration and Production.
The study was supported by the Department of Defense, the Air Force Office of Scientific Research, the Government of India Nano Mission and the state of Nuevo Leon, Mexico. Supercomputing resources were provided by Rice’s National Science Foundation-supported DAVinCI supercomputer administered by the Center for Research Computing and acquired in association with Rice’s Ken Kennedy Institute for Information Technology.
Crushing produces graphene pellets