Apr 5 2018
Scientists at the RMIT University have created an innovative artificial enzyme that uses light to destroy bacteria. This artificial enzyme can someday be used in fighting against infections, and to maintain high-risk public spaces like hospitals free of bacteria such as Escherichia coli and Golden Staph. E. coli infection can lead to gastroenteritis and dysentery. Golden Staph is the major contributor to hospital-acquired secondary infections and chronic wound infections.
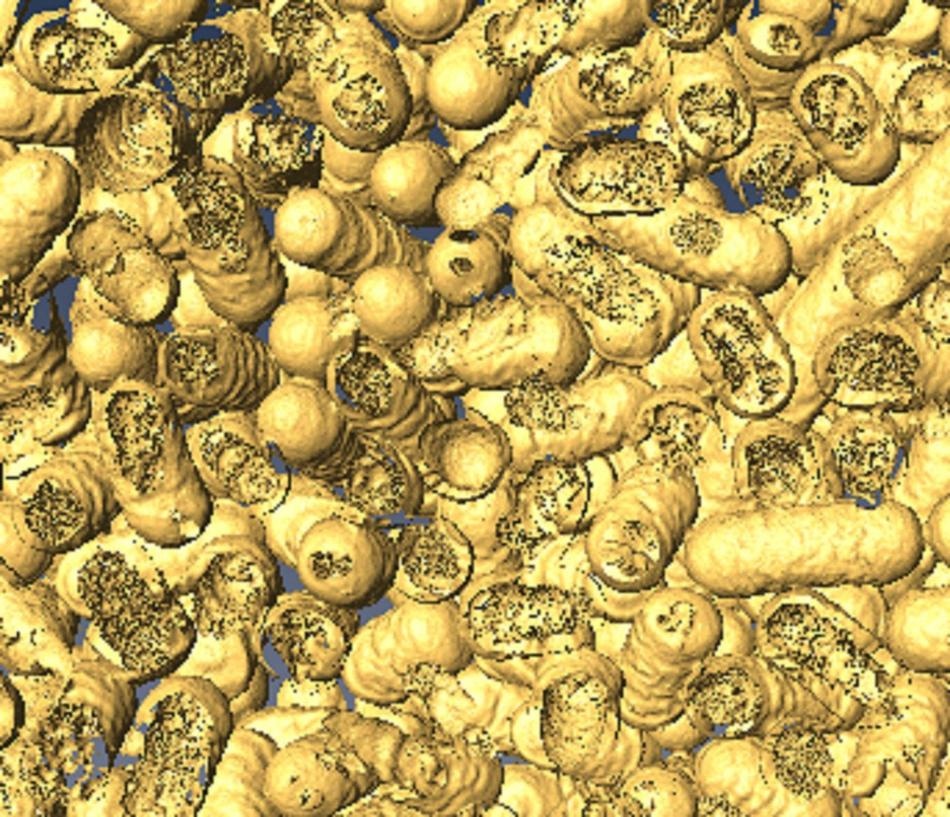 A 3D rendering of dead bacteria after it has come into contact with the NanoZymes. (Image credit: Dr. Chaitali Dekiwadia/RMIT Microscopy and Microanalysis Facility)
A 3D rendering of dead bacteria after it has come into contact with the NanoZymes. (Image credit: Dr. Chaitali Dekiwadia/RMIT Microscopy and Microanalysis Facility)
The innovative “NanoZymes” are made from tiny nanorods, with a thickness 1000 times lesser than the thickness of a strand of human hair, and use visible light to develop highly reactive oxygen species that quickly break down and destroy the bacteria.
Professor Vipul Bansal, the lead author of the study, who is an Australian Future Fellow and Director of RMIT’s Sir Ian Potter NanoBioSensing Facility, stated that the new NanoZymes are a major advancement over nature’s potential to destroy bacteria.
“For a number of years we have been attempting to develop artificial enzymes that can fight bacteria, while also offering opportunities to control bacterial infections using external ‘triggers’ and ‘stimuli’,” stated Bansal. “Now we have finally cracked it.”
“Our NanoZymes are artificial enzymes that combine light with moisture to cause a biochemical reaction that produces OH radicals and breaks down bacteria. Nature’s antibacterial activity does not respond to external triggers such as light.”
“We have shown that when shined upon with a flash of white light, the activity of our NanoZymes increases by over 20 times, forming holes in bacterial cells and killing them efficiently.”
“This next generation of nanomaterials are likely to offer new opportunities in bacteria-free surfaces and controlling the spread of infections in public hospitals.”
The NanoZymes function in a solution that is similar to the fluid in a wound, where the solution can be sprayed onto surfaces.
The NanoZymes can be also synthesized in the form of powders to be blended with ceramics, paints, and other consumer products. This relates to surfaces and walls free of bacteria in hospitals.
Public toilets—places that have higher levels of bacteria and specifically E. coli—are also a main location for the NanoZymes. The scientists are confident that their innovative technology might even have the ability to develop self-cleaning toilet bowls.
Although at present the NanoZymes use visible light from torches or similar light sources, eventually they can be activated by sunlight.
The scientists have demonstrated that the NanoZymes can function in a lab environment. At present, they are evaluating the long-term performance of the NanoZymes in consumer products.
The next step will be to validate the bacteria killing and wound healing ability of these NanoZymes outside of the lab.
This NanoZyme technology has huge potential, and we are seeking interest from appropriate industries for joint product development.
Professor Vipul Bansal
The NanoZyme advancement has recently been reported in the ACS Applied Nano Materials journal.