Nov 12 2018
A research team has come up with an easy and cost-effective method for producing irradiated nanodiamonds and other similar nanomaterials that can be used in extremely sensitive diagnostics of various diseases, including different types of cancer.
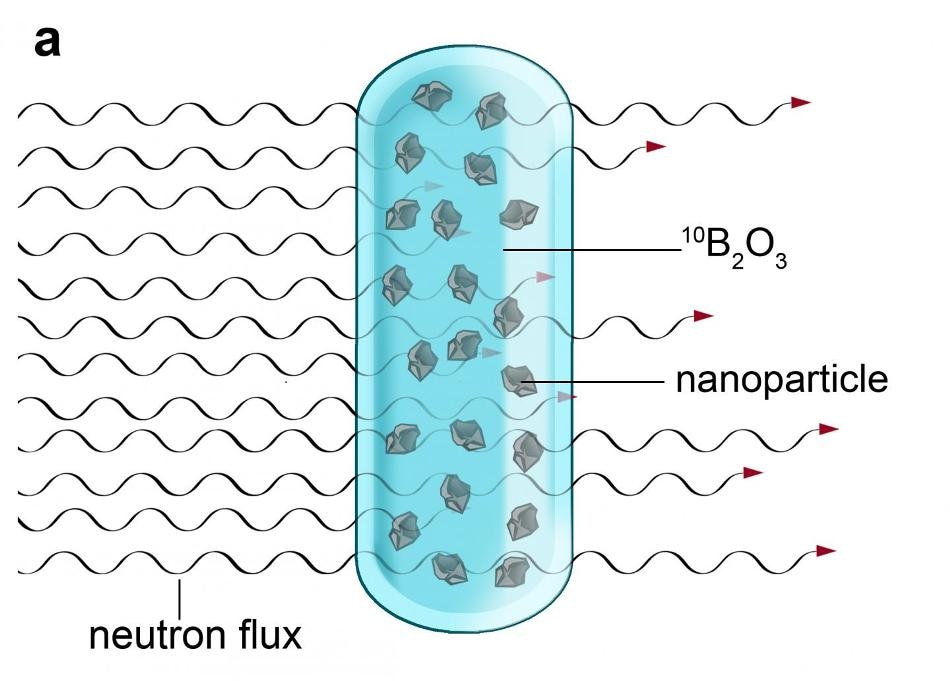 The nanocrystals must first be dispersed in molten boron oxide and then subjected to neutron irradiation in a nuclear reactor. (Image credit: IOCB Prague)
The nanocrystals must first be dispersed in molten boron oxide and then subjected to neutron irradiation in a nuclear reactor. (Image credit: IOCB Prague)
The study was headed by Petr Cígler from the Institute of Organic Chemistry and Biochemistry (IOCB Prague), and also by Martin Hrubý from the Institute of Macromolecular Chemistry (IMC), both of which are part of the Czech Academy of Sciences. The article was recently reported in the scientific journal Nature Communications.
Sensitive and selective diagnostic instruments are required for diagnosing diseases and inferring the processes that occur inside the cells at the molecular level. The presence of crystal defects in the particles of specific inorganic materials has now enabled researchers to track electric and magnetic fields in cells with excellent sensitivity and at a resolution of several dozen nanometers. Diamond is an almost perfect material for these kinds of purposes. When compared to the diamonds utilized in jewelry, the ones meant for use in diagnostics and nanomedicine, such as nanodiamonds, are about a million times smaller and are synthetically created from graphite at high temperatures and pressures.
However, a pure nanodiamond doesn’t expose much about its environment. In order to create unique defects—the so-called nitrogen-vacancy centers—which allow for optical imaging, the crystal lattice of the nanodiamond has to be damaged under regulated conditions. To create this damage, nanodiamonds are typically irradiated with fast ions in particle accelerators. These accelerated ions have the ability to displace carbon atoms from a nanodiamond’s crystal lattice, leaving behind holes called vacancies, which subsequently bond with nitrogen atoms at extreme temperatures. These nitrogen atoms exist in the crystal as contaminants. The nitrogen-vacancy centers, thus formed, are a source of fluorescence, which can be subsequently noticed. This fluorescence makes nanodiamonds extremely viable for use in technology and medicine.
However, when it comes to applying these materials on a wider scale, a major limitation is faced—that is, poor efficiency and the high cost of irradiating ions in an accelerator, which prevents the large-scale production of this highly valuable material.
The scientists from a number of research centers led by Martin Hrubý and Petr Cígler have now published an article in the journal Nature Communications in which they explained a whole new approach on how to irradiate nanocrystals. Instead of the time-intensive and expensive irradiation in an accelerator, the researchers leveraged irradiation in a nuclear reactor, which is not only relatively faster but is also much less costly.
However, it wasn’t as simple as that. The researchers had to use a trick—neutron irradiation in the reactor divides boron atoms into very fast and light ions of lithium and helium. First, the nanocrystals have to be dispersed in molten boron oxide and subsequently exposed to neutron irradiation inside a nuclear reactor. When boron nuclei capture neutrons, a thick shower of lithium and helium ions are produced which have the same impact within the nanocrystals as the ions created in a particle accelerator: the controlled generation of crystal defects. The application of a reactor to irradiate a much larger amount of material combined with the high density of the particle shower implies that it is much more economical and easier to create dozens of grams of rare nanomaterial simultaneously, which is about one thousand times more than researchers have so far been able to achieve through similar irradiation in accelerators.
The technique has been shown to be effective in the creation of defects in nanodiamonds’ crystal lattice as well as in silicon carbide—another nanomaterial. Due to this reason, researchers have hypothesized that the novel technique may be commonly used in the large-scale development of nanoparticles that have defined defects.
The latest approach employs the principle used in boron neutron capture therapy, or BNCT, in which a boron compound is administered to patients. After this compound has collected in the tumor, the patients are exposed to radiation therapy with neutrons, which divide the boron nuclei into lithium and helium ions. These ions subsequently kill the cancer tumor cells that have been accumulated by the boron. So far, this principle taken from experimental cancer therapy presents new opportunities towards the efficient development of nanomaterials that have unique potential for use in cancer diagnostics, among other fields.