Jan 25 2019
Solar rays are an abundant, clean source of energy that is becoming more and more important as the world strives to move away from power sources that lead to global warming. However, existing techniques of yielding solar charges are costly and inefficient—with a theoretical efficiency limit of 33%.
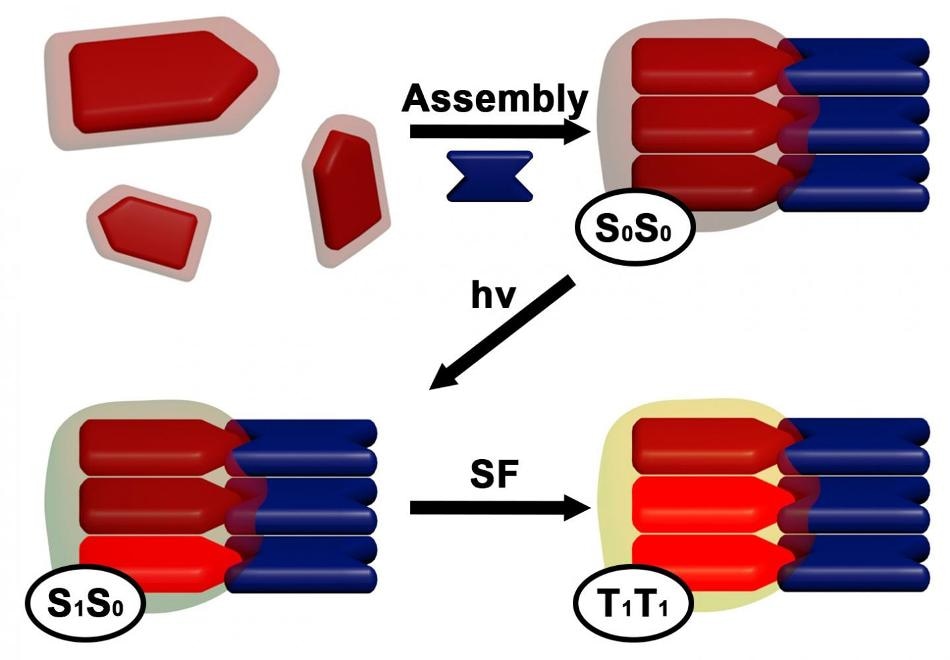 In this illustration, DPP and rylene dye molecules come together to create a self-assembled superstructure. Electrons within the structure absorb and become excited by light photons, and then couple with neighboring electrons to share energy and create additional excited electrons that can be harvested to create solar cells. (Image credit: Andrew Levine)
In this illustration, DPP and rylene dye molecules come together to create a self-assembled superstructure. Electrons within the structure absorb and become excited by light photons, and then couple with neighboring electrons to share energy and create additional excited electrons that can be harvested to create solar cells. (Image credit: Andrew Levine)
Scientists at the Advanced Science Research Center (ASRC) at The Graduate Center of The City University of New York (CUNY) have created new nanomaterials that could offer a pathway to potentially affordable and more efficient harvesting of solar energy.
The materials which the researchers developed with the ASRC’s Nanoscience Initiative produce and prolong the life of harvestable light-generated electrons using a process known as singlet fission. The finding is reported in a newly published paper in the Journal of Physical Chemistry. Previous research suggests these materials have the ability to generate more usable charges and improve the theoretical efficiency of solar cells up to 44%.
We modified some of the molecules in commonly used industrial dyes to create self-assembling materials that facilitate a greater yield of harvestable electrons and extend the electrons' excited-state lifetimes, giving us more time to collect them in a solar cell.
Andrew Levine, PhD Student, The Graduate Center, The City University of New York.
Andrew Levine is also the lead author of the paper.
Levine described that the self-assembly process causes the dye molecules to pile up in a specific fashion. This pile-up enables dyes that have absorbed solar photons to couple and share energy with—or “excite”—adjacent dyes. The electrons in these dyes then decouple in order to be obtained as harvestable solar energy.
Methodology and Findings
Scientists blended various versions of two commonly used industrial dyes—rylene and diketopyrrolopyrrole (DPP)—to produce the materials. This led to the formation of six self-assembling superstructures, which was examined by the researchers with the help of advanced spectroscopy and electron microscopy. They discovered that each combination had slight differences in geometry that impacted the excited states of the dyes, the lifetime and yield of harvestable electrons, and the occurrence of singlet fission.
Significance
This work provides us with a library of nanomaterials that we can study for harvesting solar energy. Our method for combining the dyes into functional materials using self-assembly means we can carefully tune their properties and increase the efficiency of the critical light-harvesting process.
Professor Adam Braunschweig, Associate Professor, ASRC Nanoscience Initiative and Chemistry Departments at Hunter College and The Graduate Center.
Professor Adam Braunschweig is also the lead researcher of the study.
The researchers stated that the potential of the materials to self-assemble could also reduce the time needed for producing commercially viable solar cells and prove more affordable when compared to existing fabrication techniques, which depend on the time-consuming process of molecular synthesis.
Developing a technique to harvest the solar charges produced by the new nanomaterials is the next challenge to the research team. Presently, they are working to develop a rylene molecule that can accept the electron from the DPP molecule following the singlet fission process. If successful, these materials would start the singlet fission process as well as enable charge-transfer into a solar cell.