Feb 8 2019
Germanene is a 2D material that is obtained from germanium and is associated with graphene. Since it is unstable outside the vacuum chambers where it is developed, no real measurements of its electronic properties have been performed.
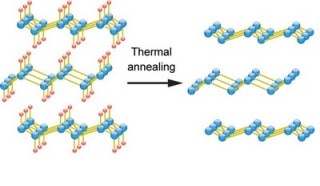 Germanane converts into germanene by thermal annealing, which removes the hydrogen (red). (Image credit: Ye Lab/University of Groningen)
Germanane converts into germanene by thermal annealing, which removes the hydrogen (red). (Image credit: Ye Lab/University of Groningen)
Researchers headed by Professor Justin Ye of the University of Groningen have now been able to develop devices with stable germanene. Basically, the material is an insulator; when heated moderately, it becomes a semiconductor, and when heated strongly, it becomes a very good metallic conductor. The outputs were reported in the journal Nano Letters on January 24th, 2019.
Materials with a single atomic layer are preferred in the construction of new types of microelectronics. Graphene, the most popular among these, is a very good conductor. Also, materials such as germanium and silicon could be exciting, since they are fully compatible with well-established protocols for device fabrication, and could be effortlessly incorporated into the existing semiconductor technology.
Unstable
But the 2D version of germanium, germanene, is very unstable.
Justin Ye, Associate Professor of Device Physics, University of Groningen.
Germanene is derived from germanium by combining calcium. The calcium ions produce 2D layers from a 3D crystal and are subsequently replaced by hydrogen. These 2D layers of hydrogen and germanium are called germanane. However, if the hydrogen is eliminated to form germanene, the material becomes unstable.
Ye and his team found a solution to this in a surprisingly simple way. They developed devices with stable germanane and then removed the hydrogen by heating the material. This produced stable devices with germanene, which enabled the researchers to study its electronic properties.
“The initial material was an insulator,” says Ye. A PhD student from his team heated these devices, which is a tried and tested technique to improve conductivity. He noticed that the material turned out to be very conductive, and its resistance was just one order of magnitude greater than graphene. “So it became an excellent metallic conductor.” Further experiments demonstrated that moderate heating (up to 200 °C) resulted in semiconducting germanane.
Hydrogen
Hence, germanene can be an insulator, a semiconductor, or a metallic conductor, based on the heat treatment to which it is subjected. Even after cooling it to room temperature, germanene remains stable. Due to heating, multilayer flakes of germanene become thinner, which proves that the change in conductivity is most probably due to the evaporation of hydrogen.
Germanene could be a material of interest in developing spintronic devices. These devices employ a current of electron spins. This is a quantum mechanical property of electrons, which can be best assumed as electrons rotating in their own axis, causing them to act like small compass needles. Although graphene is an excellent conductor of electron spins, it is difficult to control spins in this material due to their weak interaction with the carbon atoms (spin-orbit coupling).
Spintronic
“The germanium atoms are heavier, which means there is a stronger spin-orbit coupling,” says Ye. This would offer better control of spins. Since it has the ability to develop metallic germanene with excellent conductivity as well as strong spin-orbit coupling, it should consequently set the path to spintronic devices.