Feb 11 2019
Two years ago, when Hossein Robatjazi, a Rice University chemist and engineer, intended to combine a molecular sieve known as metal-organic framework (MOF) with a plasmonic aluminum nanoparticle, he never thought the outcome would be the same process used by nature to petrify wood.
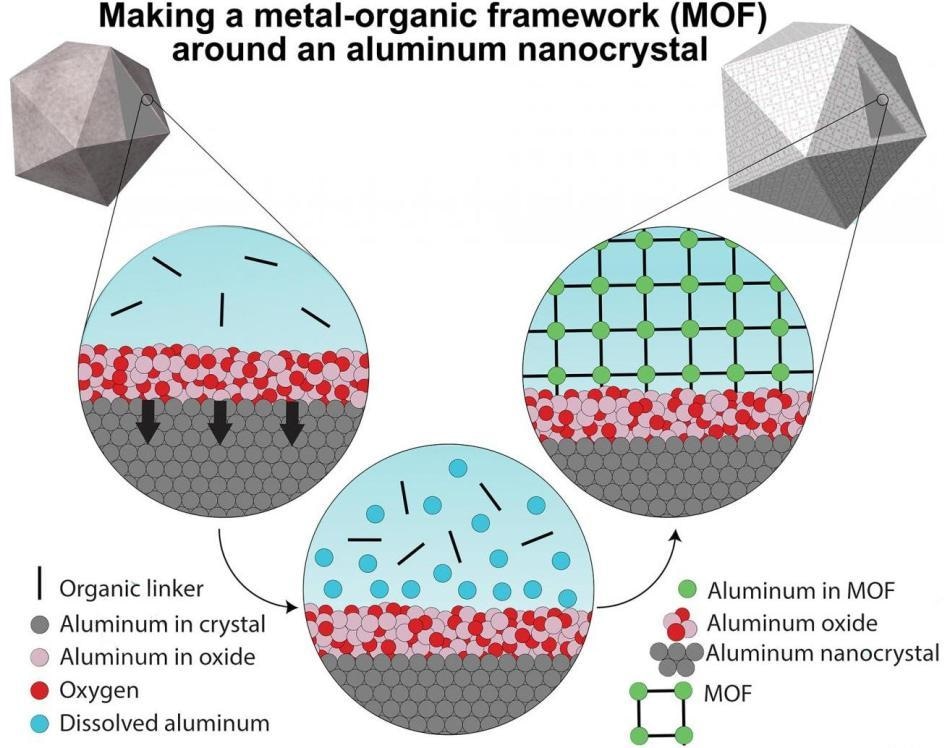 A graphic illustrating the synthesis method that begins with oxide-covered aluminum nanocrystals (top left) and ends with the nanocrystal encased in metal-organic framework (MOF). The MOF self-assembles around the particle when the oxide partially dissolves, releasing aluminum ions that bind with organic linkers to form a 3D framework. (Image credit: LANP/Rice University)
A graphic illustrating the synthesis method that begins with oxide-covered aluminum nanocrystals (top left) and ends with the nanocrystal encased in metal-organic framework (MOF). The MOF self-assembles around the particle when the oxide partially dissolves, releasing aluminum ions that bind with organic linkers to form a 3D framework. (Image credit: LANP/Rice University)
In a new paper published online in the Science Advances journal this week, Robatjazi and co-authors with Rice’s Laboratory for Nanophotonics (LANP) have detailed the way in which pseudomorphic replacement, the same chemical process by which a tree is turned into stone, helped their synthesis of the first MOF around light-powered aluminum nanocatalysts.
Catalysts are materials used to accelerate chemical reactions without reacting themselves. They are used in the production of a majority of the commercially synthesized chemicals. Since the majority of industrial catalysts function best at high pressure or high temperature or both, they are also accompanied by a huge energy burden. The combination of plasmonic aluminum and MOFs open the door for designing newer and greener catalysts that use solar energy and are developed from the most copious metal in Earth’s crust.
In the paper, Robatjazi, LANP Director Naomi Halas, and teammates carried out a proof-of-concept demonstration of a process called the reverse water-gas shift reaction at ambient pressure and temperature under laboratory conditions that mimicked sunlight. The reaction converts hydrogen gas and carbon dioxide (CO2) into carbon monoxide—a feedstock for chemical manufacturing—and water.
“This is the first example showing that you can combine MOF and aluminum particles to do this reaction with light,” stated Robatjazi, a graduate student at LANP, the Rice lab that has pioneered plasmonic technologies for applications as wide-ranging as cancer diagnosis and treatment, solar water distillation, and MRI contrast agents.
Plasmons are waves of electrons that spill over the surface of small metal nanoparticles, and by altering the size and shape of a plasmonic nanoparticle, LANP researchers can tweak it to interact with and tap energy from light. In an earlier study, LANP established copper nanocatalysts for producing clean-burning hydrogen from ammonia, and aluminum-based antenna-reactors for the production of ethylene, the chemical feedstock for polyethylene—the most common plastic in the world.
According to Halas, the most recent study with MOFs is significant for various reasons.
We’ve shown that growing MOFs around aluminum nanocrystals enhances the photocatalytic activity of the aluminum particles and also provides us a new way of controlling the size, and therefore the plasmonic characteristics, of the particles themselves. Finally, we’ve shown that the same basic method works for making different kinds of MOFs.
Naomi Halas, Director, Laboratory for Nanophotonics, Rice University
MOFs, which are three-dimensional structures, self-assemble as a result of the interaction of metal ions with organic molecules known as linkers. Similar to a sponge or Swiss cheese, the structures are extremely porous. The surface area of just a gram of some MOFs is larger than a football field, and chemists can develop MOFs that have different pore sizes, structures, and functions, such as trapping particular molecules, by altering the metal type, the linker, and the reaction conditions. Over 20,000 types of MOFs have been produced.
In the initial experiments performed by Robatjazi, he made efforts to develop MIL-53, a well-analyzed MOF that is well known for its CO2-trapping potential. He examined synthesis techniques that had worked for developing MOFs around gold particles; however, they did not work for aluminum, and Robatjazi presumed that it was due to aluminum oxide.
In contrast to gold, aluminum reacts well with oxygen, and every aluminum nanoparticle is instantly covered with a thin 2- to 4-nm sheen of aluminum oxide as soon as it comes in contact with air.
“It’s amorphous,” stated Robatjazi. “It’s not like a flat surface with a well-defined crystallinity. It’s like a bumpy road, and the MOF crystals could not make a structure on that surface.”
By observing the chemical literature, Robatjazi developed the concept of using pseudomorphic mineral replacement for preparing the surface of the particles to accept MOFs and for providing the metal building blocks for MOFs.
We learned from Mother Nature, and we basically use the same strategy because aluminum oxide is a mineral. Normally for MOFs, we mix a metal ion with the organic linker, and in this case we eliminated the metal ion and instead dissolved the aluminum oxide and used the aluminum ions from that reaction as metal components of our MOF.
Hossein Robatjazi, Chemist and Engineer, Rice University
Robatjazi discovered that the amount of aluminum surface that can be etched away can be controlled by modifying the reaction conditions, thereby controlling the final size—as well as the plasmonic properties—of the plasmonic particle within. In the case of MIL-53, the CO2-trapping MOF, he demonstrated that there was a considerable increase in the catalytic activity of the plasmonic aluminum nanocrystal when the MOF was in place.
Lastly, he showed that it is possible to use the same etching technique with different linkers, producing MOFs with different pore sizes and other properties, including a hydrophilic type that kept water away from the aluminum particle inside.
We’re exploring avenues to tune the characteristics of aluminum-MOF structures, either by synthetic variation or post-synthesis modification. That flexibility could open a range of opportunities for scaling up plasmon-mediated chemical reactions that are both less expensive for industry and better for the environment.
Naomi Halas, Director, Laboratory for Nanophotonics, Rice University
The Welch Foundation, the Air Force Office of Scientific Research, the Defense Threat Reduction Agency, the National Science Foundation, and the Rice Department of Chemistry supported the study.