Feb 26 2019
A novel genetic tool recently developed by MIT researchers can possibly make it easier to create plants that can resist fungal infections and withstand drought.
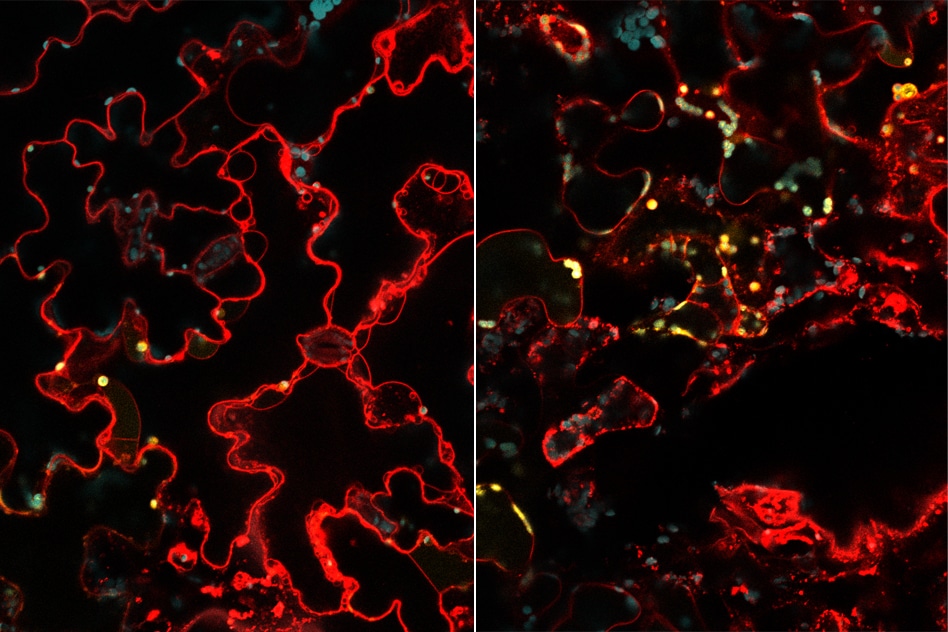 MIT engineers have developed a way to deliver genes to the chloroplasts of plant cells. In these images, mesophyll cells (right) and epidermal cells (left) in an arugula leaf have been engineered to express a yellow fluorescent protein in their chloroplasts. Cell walls are stained red and chloroplasts are stained blue. (Image credit: Tedrick Thomas Salim Lew and Seon-Yeong Kwak)
MIT engineers have developed a way to deliver genes to the chloroplasts of plant cells. In these images, mesophyll cells (right) and epidermal cells (left) in an arugula leaf have been engineered to express a yellow fluorescent protein in their chloroplasts. Cell walls are stained red and chloroplasts are stained blue. (Image credit: Tedrick Thomas Salim Lew and Seon-Yeong Kwak)
The researchers; method, in which nanoparticles are used to deliver genes within the chloroplasts of plant cells, functions with a wide range of plant species, such as spinach and other kinds of vegetables. This latest method could allow plant biologists to resolve the challenges associated with genetically modifying plants, which is currently a time-intensive and complex process that needs to be adapted to the particular plant species that is being modified.
“This is a universal mechanism that works across plant species,” stated Michael Strano, the Carbon P. Dubbs Professor of Chemical Engineering at MIT, regarding the novel technique.
The study’s senior authors are Strano and Nam-Hai Chua, the deputy chair of the Temasek Life Sciences Laboratory at the National University of Singapore and also a professor emeritus at Rockefeller University. The study has been reported in the issue of Nature Nanotechnology on February 25th, 2019.
This is an important first step toward chloroplast transformation. This technique can be used for rapid screening of candidate genes for chloroplast expression in a wide variety of crop plants.
Nam-Hai Chua, Deputy Chair, Temasek Life Sciences Laboratory, National University of Singapore
This is the first-ever study to emerge from the newly launched Singapore-MIT Alliance for Research and Technology (SMART) program in Disruptive and Sustainable Technologies for Agricultural Precision (DiSTAP), led by Chua and Strano. The study’s lead authors are Seon-Yeong Kwak, former MIT postdoc and currently the scientific director of the DiSTAP program, and Tedrick Thomas Salim Lew, a graduate student at MIT. Scientists from Yield10 Bioscience were part of the research team.
Targeting Chloroplasts
Several years ago, Strano along with colleagues found that the nanoparticles can possibly be designed to penetrate the cell membranes of plants by altering the size and electrical charge of these particles. This mechanism—referred to as lipid exchange envelope penetration (LEEP)—enabled them to engineer plants that glow, and they achieved this by integrating nanoparticles carrying a light-emitting protein, called luciferase, within their leaves.
Once the MIT researchers reported that the novel LEEP mechanism was used to embed nanoparticles into plants, plant biologists started asking whether this method could be applied to genetically engineer plants, and more particularly, to embed genes into chloroplasts. Dozens of chloroplasts are present in plant cells and therefore, prompting the chloroplasts—rather than only the nucleus—to express genes can possibly provide a means to produce relatively more amounts of a preferred protein.
Bringing genetic tools to different parts of the plant is something that plant biologists are very interested in. Every time I give a talk to a plant biology community, they ask if you could use this technique to deliver genes to the chloroplast.
Michael Strano, the Carbon P. Dubbs Professor, Department of Chemical Engineering, MIT
Commonly referred to as the site of photosynthesis, the chloroplast contains approximately 80 genes, which code for proteins needed to carry out photosynthesis. In addition, the chloroplast comes with its own ribosomes, which enable it to organize proteins inside the chloroplast. To date, researchers had found it extremely difficult to integrate genes inside the chloroplast, and the one and only existing method required applying a high-pressure “gene gun” to push genes inside the cells, which can cause damage to the plant and hence not extremely efficient.
Applying their latest technique, the MIT researchers engineered nanoparticles comprising of carbon nanotubes enclosed in a naturally occurring sugar called chitosan. The negatively charged DNA adheres loosely to the carbon nanotubes, which are positively charged. In order to embed the nanoparticles within the plant leaves, the team injects a needleless syringe containing the particle solution to the lower side of the surface of the leaf. Particles subsequently penetrate the leaf via small pores known as stomata, which usually regulate the process of water evaporation.
As soon as the nanoparticles are within the leaf, they travel via the plant cell wall, cell membranes, and finally the chloroplast’s double membranes. Once the particles penetrate into the chloroplast, the DNA is released from the nanoparticles because the chloroplast has a slightly less acidic environment. Once this DNA is free, it can be translated into proteins.
In this analysis, the investigators successfully delivered a gene for a yellow fluorescent protein, enabling them to easily view the types of plant cells that are able to express the protein. The team discovered that the protein was produced by roughly 47% of the plant cells; however, this can be possibly increased by delivering more particles, believe the researchers.
The approach reported here certainly opens new research avenues in chloroplast-selective gene delivery for transgene expression in plants, as shown here for several mature non-model species.
Sanjay Swarup, Associate Professor, Department of Biological Sciences, National University of Singapore
Swarup was not involved in the study.
More Resilient Plants
A key benefit of this method is that it can be applied across many different types of plant species. In this analysis, the team tested the new approach in watercress, arugula, spinach, tobacco, and Arabidopsis thaliana, a kind of plant often employed in studies. The researchers also demonstrated that the method is not restricted to carbon nanotubes and can possibly be extended to other different types of nanomaterials.
According to the team, plant biologists could use this novel tool to engineer a wide range of desirable qualities into crops and vegetables, in a more easy way. For instance, agricultural scientists in Singapore and elsewhere are keen on developing crops and leafy vegetables that can grow at greater densities, for urban farming.
Other promising applications include engineering crops like coffee, citrus, and bananas to be resistant to fungal infections that are likely to destroy them completely; producing drought-resistant crops; and modifying crops like rice so that they do not absorb arsenic from groundwater.
Since the engineered genes are only carried in the chloroplasts, which are maternally inherited, they cannot be transmitted to other types of plant species and thus can only be passed to offspring.
That’s a big advantage, because if the pollen has a genetic modification, it can spread to weeds and you can make weeds that are resistant to herbicides and pesticides. Because the chloroplast is passed on maternally, it’s not passed through the pollen and there’s a higher level of gene containment.
Tedrick Thomas Salim Lew, Graduate Student, MIT
The National Research Foundation of Singapore and the Singapore-MIT Alliance for Research and Technology Center funded the study.