Sep 2 2019
Previously, the idea of tiny nanobots that flow through the body to rectify damaged cells was regarded as science fiction. However, these microrobots are now turning into reality with a lot of experimental trials.
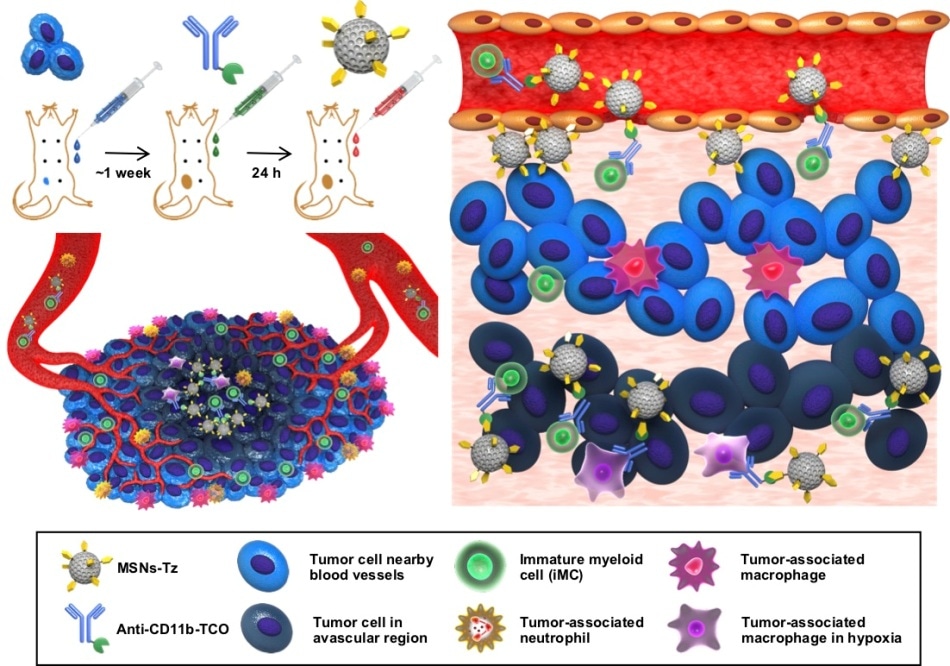 A schematic representation of click reaction-assisted immune cell targeting (CRAIT) strategy used to enhance tumor penetration of drug-loaded NPs. (Left, top) Antibodies are pre-injected to label circulating immune cells, and drug-loaded nanoparticles are subsequently administered to target the immune cells via click reaction. (Left, bottom) Schematic illustration of tumor microenvironment featuring inflammatory cell recruiting and inhomogeneous blood vessel distribution. (Right) Schematic illustration of the principles of CRAIT strategy. Immune cells are labeled by antibodies and subsequently tagged with nanoparticles by a click reaction. The labeled cells transport nanoparticles from tumor periphery to tumor interior. (Image credit: Institute for Basic Science)
A schematic representation of click reaction-assisted immune cell targeting (CRAIT) strategy used to enhance tumor penetration of drug-loaded NPs. (Left, top) Antibodies are pre-injected to label circulating immune cells, and drug-loaded nanoparticles are subsequently administered to target the immune cells via click reaction. (Left, bottom) Schematic illustration of tumor microenvironment featuring inflammatory cell recruiting and inhomogeneous blood vessel distribution. (Right) Schematic illustration of the principles of CRAIT strategy. Immune cells are labeled by antibodies and subsequently tagged with nanoparticles by a click reaction. The labeled cells transport nanoparticles from tumor periphery to tumor interior. (Image credit: Institute for Basic Science)
In general, it is considered that nanoparticles are so small that they roam freely all through the body after being administered, but this is only partially true.
Nanoparticles can enter tumors only to a depth of 100 µm from the vessels. Many barriers such as inhomogeneous vascular distribution, dense tumor tissue, and high interstitial pressure can also hamper the diffusion of the nanoparticles. Hence, cancer cells deep within the tissue may live, causing recurrence.
Astonishingly, it has been said that immune cells accumulate at deep tumors. When tumors outgrow the supply of blood, immune cells are ideally transferred to a tumor microenvironment to support the blood supply to tumors and tissue remodeling. Many efforts have been made to use immune cells to administer anti-cancer drugs to the areas inaccessible by traditional targeting methods.
A majority of them necessitate time-intensive manipulations to extract, grow, and inject cells; hence, this ex vivo process minimizes treatment efficacy. Others investigated ways to enable the antibody that carries nanoparticles to target immune cells. Even this method was found to be ineffective since nanoparticles bulk up with the chemotherapy drug carried and cannot reach the targeted spot efficiently.
In a study published in Journal of the American Chemical Society, the collaborative research team headed by Director Taeghwan Hyeon at the Center for Nanoparticles of the Institute for Basic Science (IBS) in Daejeon, Dr Seung-Hae Kwon at Korea Basic Science Institute in Seoul, and Prof. Nohyun Lee at Kookmin University in Seoul, South Korea reported an innovative targeting approach that enables deep tumor penetration of drug-carrying nanoparticles.
They employed a “click reaction”, which is a chemical reaction that effortlessly combines molecular building blocks similar to two pieces of a seat belt that “click” to get buckled.
Our idea was to induce the linking of immune cell-targeting antibodies to drug-loaded nanoparticles on the cells, instead of taking them up in the cells or using antibody-nanoparticle conjugates. Most other studies did so and failed to produce satisfactory results.
Nohyun Lee, Study Corresponding Author, Professor, Institute for Basic Science
In a click reaction, chemical reagents facilitate an easy link of unnatural chemical groups to any region of a target protein with high site-selectivity. As part of the study, scientists used the click reaction between tetrazine and trans-cyclooctene.
To label tumor-infiltrating immune cells, trans-cyclooctene-functionalized antibodies are administered into mice. After some time, tetrazine-functionalized mesoporous silica nanoparticles are injected so that they “click” to get connected to immune cells.
This click reaction-assisted immune cell targeting (CRAIT) strategy successfully ‘invaded’ the intended areas: Real-time fluorescence imaging of the tumor tissue shows that motile immune cells transport the nanoparticles as seen in Figure 2. Compared to passive targeting, the CRAIT method brought a twofold reduction in the tumor burden in aggressive breast cancer models.
Dr Soo Hong Lee, Study First Author, Institute for Basic Science
The nanoparticles carrying doxorubicin, an anticancer drug, did not have any effect on the migration and viability of the cells.
According to Director Taeghwan Hyeon, the corresponding author of the study, “The intratumoral distribution of nanoparticles delivered by the CRAIT method was the key to overcoming limitations of conventional delivery methods. This study will broaden the application of nanomedicines.”
As the CRAIT technique is dependent on the click reaction, it can be used for different delivery vehicles such as liposomes, micelles, and other nanoparticles. Moreover, in case sufficient antibodies are available, a number of circulating cells can be used as delivery vehicles.
Since the circulating cells take part in different inflammatory diseases, the coverage of the CRAIT technique is not restricted to cancer. The all-around CRAIT technique is simple and necessitates modification of nanoparticles and antibodies using well-developed bio-conjugation reaction.