Mar 16 2020
Researchers at KAIST have designed a new three-dimensional (3D) hierarchically porous nanostructured catalyst that exhibits a carbon dioxide (CO2) to carbon monoxide (CO) transformation rate of up to 3.96 times higher when compared to traditional nanoporous gold catalysts.
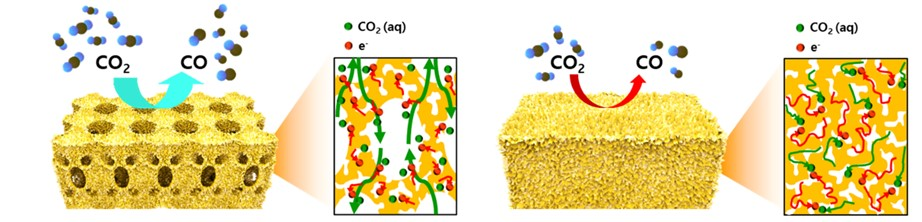 Schematic illustration and cross-sectional view with the expected reaction pathway for the hierarchically porous gold and nanoporous gold electrodes. Image Credit: Professor Seokwoo Jeon and Professor Jihun Oh, KAIST.
Schematic illustration and cross-sectional view with the expected reaction pathway for the hierarchically porous gold and nanoporous gold electrodes. Image Credit: Professor Seokwoo Jeon and Professor Jihun Oh, KAIST.
This novel catalyst assists to overcome the current drawbacks of the mass transport that has been the main reason for the reduction in the rate of CO2 conversion, thereby holding the potential for economical, large-scale electrochemical conversion of CO2 into useful chemicals.
With the increase in CO2 emissions and worldwide depletion of fossil fuels, electrochemical reduction and conversion of CO2 to clean energy has gained considerable attention as a potential technology.
Since CO2 reduction reaction takes place competitively with hydrogen evolution reactions (HER) at comparable redox potentials, creating an efficient electrocatalyst for selective and strong CO2 reduction reactions has continued to be a main technological problem.
In CO2 reduction reactions, Gold (Au) is one of the most commonly utilized catalysts. However, the limited supply and high cost of Au have been barriers to mass commercial applications. The growth of nanostructures has been widely investigated as a prospective technique to enhance the selectivity for target products and to optimize the number of active stable sites, thereby improving energy efficiency.
But, the nanopores of the complex nanostructures reported earlier were easily blocked by the gaseous CO bubbles at the time of aqueous reactions. The CO bubbles inhibited the mass transport of the reactants via the electrolyte, leading to low CO2 transformation rates.
As part of the study, which was recently reported in the Proceedings of the National Academy of Sciences of the USA (PNAS) on March 4th, 2020, a team of researchers at KAIST, guided by Professor Seokwoo Jeon and Professor Jihun Oh from the Department of Materials Science and Engineering, has developed a 3D hierarchically porous Au nanostructure with two different sizes of nanopores and macropores.
The researchers employed proximity-field electroplating and nanopatterning (PnP) methods that are very useful for fabricating the 3D well-ordered nanostructures.
The suggested nanostructure, which includes interconnected macroporous channels with a width of 200 to 300 nm and nanopores measuring 10 nm, causes efficient mass transport via the interconnected macroporous channels and ensures high selectivity by creating highly active stable sites from several nanopores.
Consequently, its electrodes exhibit a high CO selectivity of 85.8% at a low overpotential of 0.264 V and efficient mass activity ranging up to 3.96 times than that of de-alloyed nanoporous Au electrodes.
These results are expected to solve the problem of mass transfer in the field of similar electrochemical reactions and can be applied to a wide range of green energy applications for the efficient utilization of electrocatalysts.
Study Researchers, KAIST
This study was funded by the National Research Foundation (NRF) of Korea.