Mar 11 2021
Scientists have found a way to control the size of special nanoparticles to optimize their use for both magnetic resonance and near-infrared imaging. Their approach could help surgeons use the same nanoparticles to visualize tumours just before and then during surgery using the two different imaging techniques. Their findings were published in the journal Science and Technology of Advanced Materials.
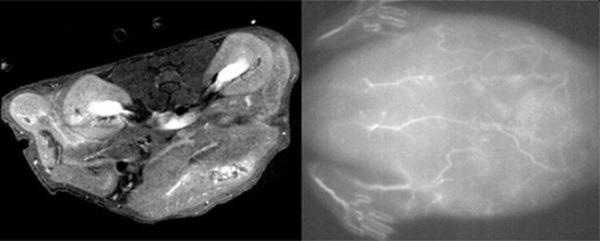 The scientists injected the nanoparticle solution into the tail veins of live mice and were able to obtain high quality MRI (left) and near-infrared fluorescence (right) scans of tissues and blood vessels. Image Credit: STAM
The scientists injected the nanoparticle solution into the tail veins of live mice and were able to obtain high quality MRI (left) and near-infrared fluorescence (right) scans of tissues and blood vessels. Image Credit: STAM
“Magnetic resonance imaging is routinely used in pre-operative diagnosis, while surgeons have started using near-infrared fluorescence imaging during surgical procedures,” says nanobiotechnologist Kyohei Okubo of Tokyo University of Science. “Our nanoparticle probes could provide a bimodality that will be clinically appealing to medical device researchers and doctors.”
Ceramic nanoparticles made with the rare earth metals ytterbium (Yb) and erbium (Er) have demonstrated low toxicity and prolonged near-infrared luminescence, showing promise as a contrast agent in MRI scans and a fluorescing agent for near-infrared fluorescence imaging. Images of blood vessels and organs in live bodies can be obtained with the two imaging techniques by further modifying the nanoparticle surfaces with polyethylene glycol (PEG)-based polymers. But to improve image resolution, scientists need to have more control over nanoparticle size during the fabrication process.
Okubo and his colleagues used a step-by-step fabrication process that starts with mixing rare earth oxides in water and trifluoracetic acid. The mixture is heated to form a solid. Then it is dissolved in solution, oleic acid is added and gas is removed. So-called rare-earth-doped ceramic nanoparticles form when this solution is cooled.
A few more steps lead to the coating of the nanoparticle surfaces with PEG. The scientists found they could slow the growth rate of the nanoparticles by increasing their concentration before the coating process. This allowed them to form nanoparticles 15 and 45 nanometres in diameter.
The team conducted a series of tests to examine the properties of their nanoparticles. They found that they could be used for obtaining high-quality images of blood vessels in live mice using MRI and near-infrared fluorescence imaging techniques. Further tests showed the nanoparticles exhibited minimal toxicity on mouse fibroblast cells when used in low concentrations. They also have a short half-life, meaning they would be cleared relatively quickly from the body, making them safe for clinical use.
The team next aims to investigate how different distributions of paramagnetic ions on the nanoparticles affect their magnetic properties. They also aim to study whether modifications made to the nanoparticles could make them applicable for use in light-based ‘photodynamic’ therapies for treating skin cancers and acne, for example.