Mar 26 2021
Generally, when crystals are grown, atoms will initially combine together into tiny clusters. This process is referred to as nucleation.
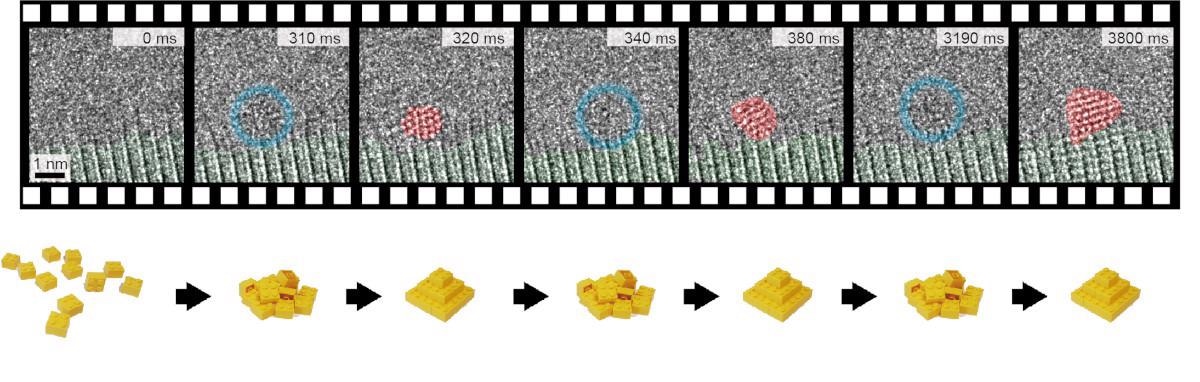 Stills from a slow-motion video of the reversible Au crystal formation process on the atomic scale. Image Credit: Lawrence Berkeley National Laboratory.
Stills from a slow-motion video of the reversible Au crystal formation process on the atomic scale. Image Credit: Lawrence Berkeley National Laboratory.
However, the precise understanding of how this atomic ordering occurs from the chaos of arbitrarily moving atoms has eluded researchers for a long time.
According to traditional nucleation theory, crystals create a single atom at a time, consistently raising the level of order. New analyses have also noted a two-step nucleation process in which a high-energy, transient structure forms initially and this structure subsequently transforms into a stable crystal.
However, according to an international research group, jointly headed by the Lawrence Berkeley National Laboratory (Berkeley Lab) of the Department of Energy, the actual story is much more complex.
The researchers’ findings have now revealed that instead of grouping together in a sequential manner or rendering a single irreversible transition, gold atoms will rather organize on their own, split up, regroup, and subsequently reorganize several times before assuming an ordered and stable crystal. The study results were recently published in the Science journal.
For the first time, the researchers were able to observe this fast and reversible nucleation process using a sophisticated electron microscope Their study offers a concrete understanding of the early phases of several growth processes, like nanoparticle formation and thin-film deposition.
As scientists seek to control matter at smaller length scales to produce new materials and devices, this study helps us understand exactly how some crystals form.
Peter Ercius, Study Co-Lead Author and Staff Scientist, Molecular Foundry, Lawrence Berkeley National Laboratory
In line with the team’s traditional understanding, as soon as the crystals reach a specific size, they do not return to an unstable, disordered state.
Won Chul Lee, one of the professors directing the study, explained this phenomenon this way: if each atom is visualized as a Lego brick, then rather than constructing a house using one brick at a time, the bricks constantly fit together and split up again until they are sufficiently strong to remain together. But after the foundation is set, additional bricks can be added without disturbing the overall structure.
In this case, the team could only visualize the unstable structures because of the speed of the recently designed detectors on the TEAM I—one of the most robust electron microscopes in the world. The experiments were guided by a group of in-house experts from the National Center for Electron Microscopy in the Molecular Foundry of the Berkeley Lab.
The researchers used the TEAM I microscope and recorded live atomic-resolution images at rates of up to 625 frames per second, which is quite fast for electron microscopy, and around 100-fold faster than earlier analyses.
Individual gold atoms were observed as they developed into crystals, broke apart into separate atoms, and subsequently reformed repeatedly into various crystal configurations before ultimately stabilizing.
Slower observations would miss this very fast, reversible process and just see a blur instead of the transitions, which explains why this nucleation behavior has never been seen before.
Peter Ercius, Study Co-Lead Author and Staff Scientist, Molecular Foundry, Lawrence Berkeley National Laboratory
The rationale behind this reversible phenomenon is that the formation of crystals is an exothermic process—in other words, it discharges energy. As a matter of fact, the precise energy discharged when atoms bind to the small nuclei can increase the local “temperature” and, thus, melt the crystal.
This way, the preliminary process of crystal formation works against itself, varying between disorder and order several times before constructing a nucleus that is sufficiently stable to tolerate the heat.
To validate this understanding of their experimental observations, the researchers performed calculations of binding reactions between a nanocrystal and a hypothetical gold atom.
Scientists are now designing detectors that are relatively faster and could perhaps be used to capture the process at higher speeds. This approach may help them figure out if there are additional characteristics of nucleation concealed in the atomic chaos.
The researchers are also hoping to detect analogous transitions in various atomic systems to find out whether this latest finding reflects a general nucleation process.
From a scientific point of view, we discovered a new principle of crystal nucleation process, and we proved it experimentally.
Jungwon Park, Study Lead Author, Seoul National University
Berkeley Lab headed the collaborative study in association with South Korea’s Hanyang University, Seoul National University, and the Institute for Basic Science.
The Molecular Foundry is a DOE Office of Science user facility.
The study was mainly funded by the National Research Foundation of Korea. The research work at the Molecular Foundry was financially supported by the U.S. Department of Energy Office of Science, Office of Basic Energy Sciences.
The Institute for Basic Science (Korea), the U.S. National Science Foundation, and Samsung Science and Technology Foundation provided additional funding.
Revealing the Nano Big Bang in Crystal Formation
Berkeley Lab scientists and collaborators took advantage of one of the best microscopes in the world—the TEAM I electron microscope at the Molecular Foundry—to watch how individual gold atoms organized themselves into crystals on top of graphene. The research team observed as groups of gold atoms formed and broke apart many times, trying out different configurations, before finally stabilizing. Video Credit: Lawrence Berkeley National Laboratory.
Journal Reference:
Jeon, S., et al. (2021) Reversible disorder-order transitions in atomic crystal nucleation. Science. doi.org/10.1126/science.aaz7555.