May 21 2021
Carbon occurs in different forms in nature. Apart from graphite and diamond, there are some recently discovered forms with surprising properties.
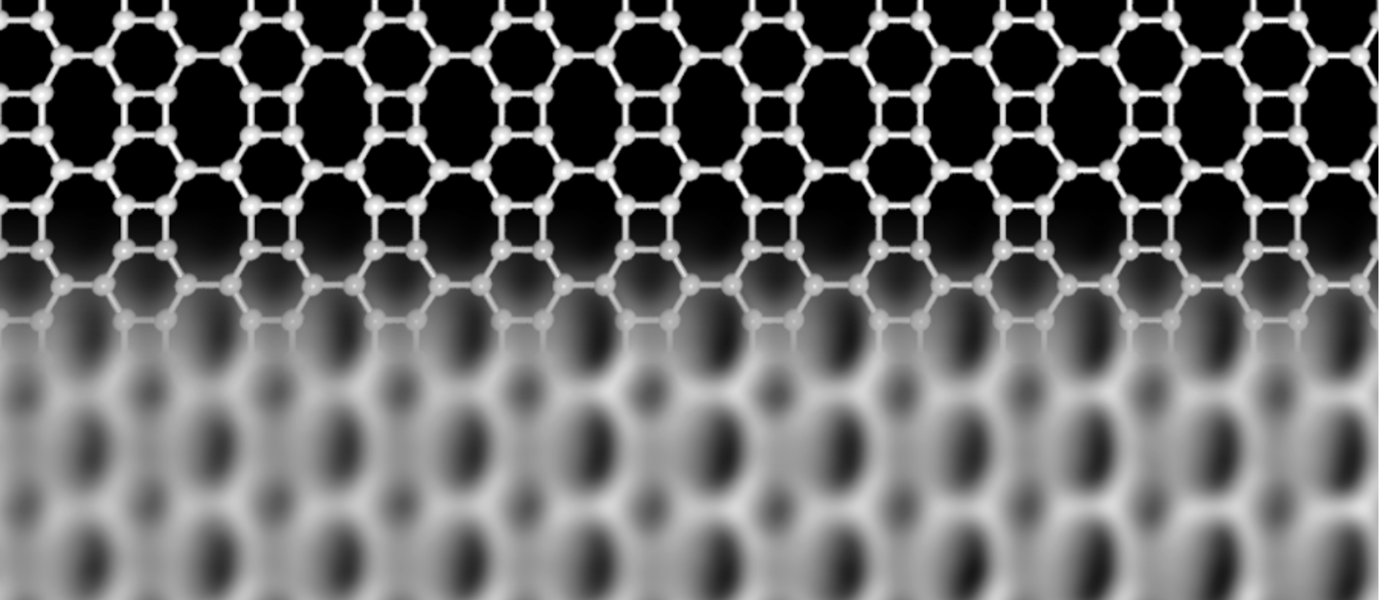 Structure of the new network. The upper part schematically shows how the carbon atoms link as squares, hexagons, and octagons. The lower part is an image of the network, obtained with high-resolution microscopy. Image Credit: University of Marburg and Aalto University.
Structure of the new network. The upper part schematically shows how the carbon atoms link as squares, hexagons, and octagons. The lower part is an image of the network, obtained with high-resolution microscopy. Image Credit: University of Marburg and Aalto University.
For instance, graphene, having a thickness of only one atomic layer, is the thinnest known material and its exceptional properties make it a highly exciting candidate for applications such as high-tech engineering and future electronics.
As far as graphene is concerned, each carbon atom is connected to three neighbors, thereby developing hexagons that are arranged in a honeycomb network.
Moreover, theoretical studies have illustrated that carbon atoms can be organized in other flat network patterns, while still being connected to three neighbors, but none of such estimated networks had been accomplished so far.
At the University of Marburg in Germany and Aalto University in Finland, scientists have currently found a new carbon network, which is atomically thin like graphene but composed of squares, hexagons, and octagons that form an ordered lattice.
The researchers verified the special structure of the network with the help of high-resolution scanning probe microscopy and intriguingly found that its electronic properties are quite different from those of graphene.
When compared to graphene and other carbon forms, the new Biphenylene network—as the new material is named—exhibits metallic properties. The network’s narrow stripes, with a width of just 21 atoms, already act like a metal, while graphene is a semiconductor of this size.
These stripes could be used as conducting wires in future carbon-based electronic devices.
Michael Gottfried, Professor, University of Marburg
Gottfried heads the team that developed the concept.
According to Qitang Fan the lead author of the study from Marburg, “This novel carbon network may also serve as a superior anode material in lithium-ion batteries, with a larger lithium storage capacity compared to that of the current graphene-based materials.”
At Aalto University, the research group helped to image the material and decode its properties.
Professor Peter Liljeroth’s team performed the high-resolution microscopy that revealed the material’s structure, while scientists headed by Professor Adam Foster employed computer analysis and simulations to comprehend the interesting electrical properties of the material.
The new material is created by putting together the carbon-containing molecules on a highly smooth gold surface. Initially, these molecules develop chains, comprising connected hexagons, and the following reaction links these chains jointly to form the octagons and squares.
An essential feature of the chains is that they are chiral, which implies that they occur in two mirroring types, like left and right hands. But only chains of the same kind accumulate on the surface of the gold, thereby developing well-ordered assemblies, before they get connected.
This is vital for the development of the new carbon material since the reaction between two different types of chains results only in graphene.
The new idea is to use molecular precursors that are tweaked to yield biphenylene instead of graphene.
Linghao Yan, Aalto University
Yan performed the high-resolution microscopy experiments at Aalto University.
For the time being, the groups work to generate bigger sheets of the material, so that its application potential can be investigated more.
We are confident that this new synthesis method will lead to the discovery of other novel carbon networks.
Peter Liljeroth, Professor, Department of Applied Physics, Aalto University
Journal Reference:
Fan, Q., et al. (2021) Biphenylene network: A nonbenzenoid carbon allotrope. Science. doi.org/10.1126/science.abg4509.