Reviewed by Alex SmithAug 26 2021
Researchers at the Chalmers University of Technology have introduced a new concept to fabricate high-performance electrode materials for sodium batteries. The work is an attempt to find sustainable energy storage.
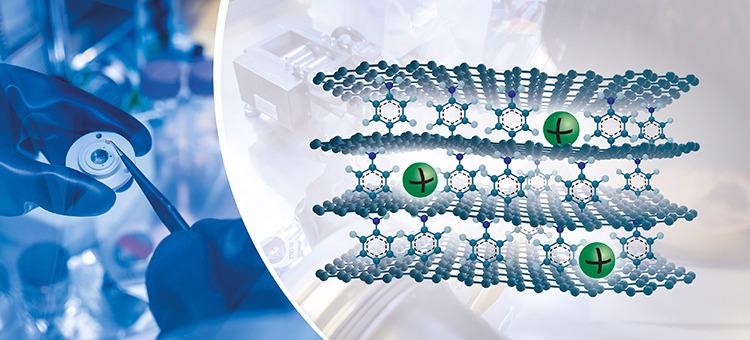 Sustainable concept. Sodium is one of the most abundant and affordable metals in the world. Now researchers at the Chalmers University of Technology present a concept that allows sodium-ion batteries to match the capacity of today's lithium-ion batteries. Using a novel type of graphene, they stacked specially designed graphene sheets with molecules in between. The new material allows the sodium ions (in green) to efficiently store energy. Image Credit: Marcus Folino and Yen Strandqvist/Chalmers University of Technology.
Sustainable concept. Sodium is one of the most abundant and affordable metals in the world. Now researchers at the Chalmers University of Technology present a concept that allows sodium-ion batteries to match the capacity of today's lithium-ion batteries. Using a novel type of graphene, they stacked specially designed graphene sheets with molecules in between. The new material allows the sodium ions (in green) to efficiently store energy. Image Credit: Marcus Folino and Yen Strandqvist/Chalmers University of Technology.
The approach is built on a novel type of graphene with the ability to store sodium, which is one of the world’s most widely used and cheapest metal ions. The outcomes revealed that the capacity matches today’s lithium-ion batteries.
Although lithium ions perform better for energy storage, lithium is a costly metal concerning its long-time supply and environmental issues.
Sodium is an abundant low-cost material and is a major constituent in seawater (and in kitchen salt). This turns sodium-ion batteries into an attractive and sustainable alternative for decreasing the requirements for critical raw materials. However, challenges lie ahead in terms of elevating the capacity.
With the present state of performance, sodium-ion batteries are unable to compete with lithium-ion cells. The limitation is graphite, which is comprised of stacked layers of graphene and employed as the anode in contemporary lithium-ion batteries.
The ions intercalate in the graphite, meaning that they tend to shift in and out of the graphite layers and enable storage for energy use. Sodium ions are bigger than lithium ions and react differently. This poses challenges in storing them efficiently in the graphite structure. However, the research presents a novel method to resolve this issue.
We have added a molecule spacer on one side of the graphene layer. When the layers are stacked together, the molecule creates larger space between graphene sheets and provides an interaction point, which leads to a significantly higher capacity.
Jinhua Sun, Study First Author and Researcher, Department of Industrial and Materials Science, Chalmers University of Technology
The scientific paper was published in the journal Science Advances.
Ten Times the Energy Capacity of Standard Graphite
Typically, the capacity of sodium intercalation in standard graphite ranges at 35 mA h g−1. This is less than one-tenth of the capacity for lithium-ion intercalation in graphite. Using the novel graphene, the specific capacity for sodium ions is 332 mA h g−1, nearing the value for lithium in graphite. The results also revealed complete reversibility and high cyclic stability.
It was really exciting when we observed the sodium-ion intercalation with such high capacity. The research is still at an early stage, but the results are very promising. This shows that it’s possible to design graphene layers in an ordered structure that suits sodium-ions, making it comparable to graphite.
Aleksandar Matic, Professor, Department of Physics, Chalmers University of Technology
“Divine” Janus Graphene Opens Doors to Sustainable Batteries
The research was started by Vincenzo Palermo in his earlier position as Vice-Director of the Graphene Flagship, a European Commission-funded project coordinated by the Chalmers University of Technology.
The novel graphene possesses asymmetric chemical functionalization on opposite faces and is, therefore, referred to as Janus graphene, after the two-faced ancient Roman God Janus — the God of new beginnings. The name is related to doors and gates, as well as the initial steps of a journey. However, here, the name Janus graphene relates to roman mythology, denoting potential gateway to high-capacity sodium-ion batteries.
Our Janus material is still far from industrial applications, but the new results show that we can engineer the ultrathin graphene sheets — and the tiny space in between them — for high-capacity energy storage. We are very happy to present a concept with cost-efficient, abundant and sustainable metals.
Vincenzo Palermo, Affiliated Professor, Department of Industrial and Materials Science, Chalmers University of Technology
The study was financially supported by the European Union’s Horizon 2020 research and innovation program under GrapheneCore3 881603–Graphene Flagship, FLAG-ERA project PROSPECT, the Chalmers Foundation and the Swedish Research Council. The calculations were performed at C3SE (Gothenburg, Sweden) through a SNIC grant. This work was performed, in part, at Myfab Chalmers and Chalmers materials analysis laboratory.
Journal Reference:
Sun, J., et al. (2021) Real-time imaging of Na+ reversible intercalation in “Janus” graphene stacks for battery applications. Science Advances. https://www.science.org/doi/10.1126/sciadv.abf0812.