Research published in the journal Talanta Open embarks on the use of a Single-atom Gold (Au) catalyst that readily enhances the selectivity phenomena of hydrogen peroxide (H2O2) sensors. This novel study demonstrates that the single-atom nanozyme detection approach may be used to sense reactive oxygen species (ROS) to a high capability.

Study: Single-atom Au catalyst loaded on CeO2: A novel single-atom nanozyme electrochemical H2O2 sensor. Image Credit: sulit.photos/Shutterstock.com
Reactive Oxygen Species and H2O2
Reactive oxygen species (ROS) is the term used for reaction products where incomplete oxygen reduction occurs, leading to the synthesis of such chemical substances via different thermo-chemical processes.
In living organisms, H2O2 is a typical ROS. Compared to other substances, there are various adverse undesirable consequences when a higher concentration is noted and persists in tissues. For example, elevated H2O2 levels are linked to memory loss, neurological damage, aging, and malignancy.
H2O2 Detection Techniques
Numerous approaches, including the fluorescence method for detection, spectrophotometric analysis, the titration technique, and electrochemical methods, have been employed in the past to sense H2O2. The electrochemical approach offers benefits, including ease of use, high responsiveness, and quick reaction time.
Because of their enzyme replicating actions, electrochemical H2O2 sensors made via nanotechnology and enzyme reactions have lately received a lot of interest.
Introduction to Single-Atom Catalysts
The nanozyme variation known as Single-atom catalyst substance is made up of segregated metal individual atoms scattered on a reference base plane. Iron, Palladium, and Gold atoms are included in the most widely utilized SACs.
SACs have significant benefits due to their exceptional electrical and morphological features, such as reduced mental expenditure and increased enzymatic performance.
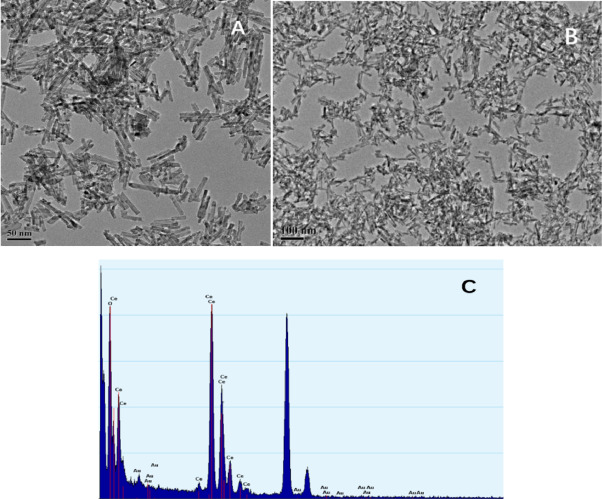
(A) and (B) HRTEM images and (C) EDS mapping of the 0.3% Au–CeO2 nanocomposite. Image Credit: Wu, J. et al.
Utilization of Single-Atom Catalysts
SAC has been the center of focus for a long time and has been utilized for various purposes owing to its advantageous properties. Owing to their unique properties, they are utilized in hydrogenation processed for various substances such as alkenes and alkynes. The enzymatic oxidation process of Carbon and Hydrogen based compounds also uses them readily. Single-atom catalysts are extensively utilized for biomedical applications such as effective use in cancer therapy. Furthermore. their effective enzymatic properties have been used for the elimination of cancer cells. A major application also includes the biosensing field because of its biocompatibility.
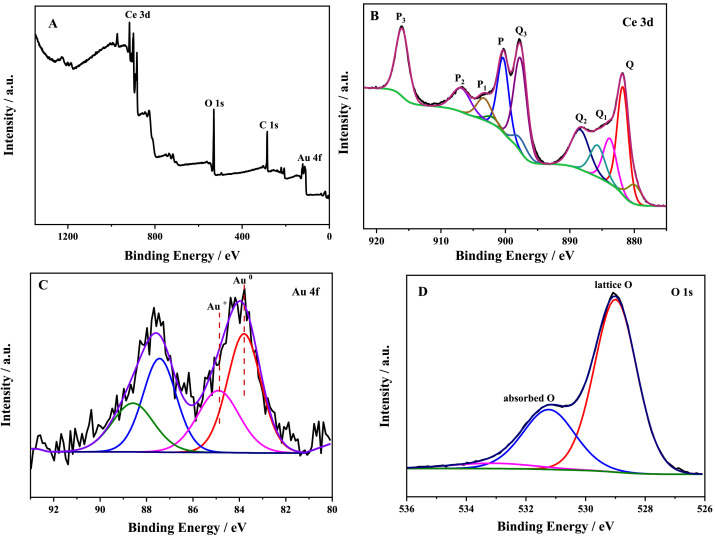
(A) XPS survey spectra and (B) high-resolution XPS spectrum of Ce 3d, (C) Au 4f, (D) O 1s of Au–CeO2. Image Credit: Wu, J. et al.
Importance of Single-Atom Au
Since stabilized Au nanocatalysts exhibit high catalytic activity, single-atom Au has been frequently employed. Although smaller Au nanoparticles exhibit excellent enzymatic activity, placing Au nanoparticles on oxide substrates causes sintering, resulting in bigger particles. It was recently revealed that single-atom Au, which has a significant catalytic impact, may be spread in an ordered way on appropriate supports.
Metal oxides, among the multiple kinds of substrates, have particular and adaptable qualities and several defect locations on their interfaces; such locations or voids can attach a solitary metal atom. The substrate for catalytic degradation of H2O2 must include oxygen vacancies along with the necessary availability of active catalyst attachment sites. For this purpose, CeO2 is the most viable and extensively used oxide.
A unique approach for detecting H2O2 generated by healthy live cells has been thoroughly investigated, employing a composite nanostructure of single-atom Au and CeO2 nanorods (Au–CeO2).
Research Findings
The researchers initially analyzed the surface morphology of the substance. The produced Au–CeO2 nanocomposite has a cylindrical rod-like molecular structure with a molecular length range of 50–150 nm and a diameter of 10 nm. The X-ray crystallography showed the cubic molecular lattice of CeO2.
Three distinct nanoparticle composites were produced and employed to alter the GCE electrode, with single-atom Au loadings of 0.2, 0.3, and 0.4 percent. The maximum electrochemical reaction to H2O2 was observed when the Au concentration was 0.3 percent, implying that the most significant amount of oxygen vacancies was produced in CeO2 at this dosage.
Optimization Study Findings
To improve the precision of this nanozyme sensing and attain the highest efficiency, control parameters must be optimized. As a result, the quantity of enzyme/catalyst modification and the pH value of the solutions were considered, and the modification concentration of 8 μ L was deemed to be optimum. A variation in this concentration led to either overproduction or inadequate synthesis on the electrode surface. Similarly, the optimization technique revealed the optimum pH value to be around 7.
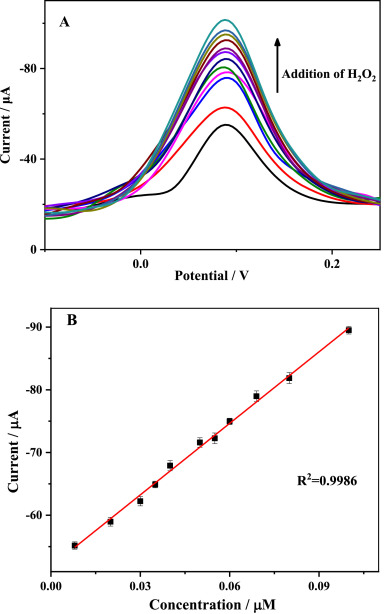
(A) DPV response obtained at the Au–CeO2/GCE with continuous addition of H2O2 in 0.1 M PBS (pH = 7.0). (B) Calibration plot for H2O2 determination. Image Credit: Wu, J. et al.
Future Industrial Impacts of the Study
The testing revealed excellent characteristic properties in terms of reproducibility, stability, and longevity. This technique would ensure a more efficient process of sensing H2O2 by cancer cells leading to a swift and efficient diagnostic method. The supersensitive property of the sensors also would lead to efficient biomedical sensors surpassing currently used instrumentation.
In short, the single-atom Au catalyst synthesis was successfully incorporated for the detection of H2O2, resulting in a substantial increase in the sensor’s effectiveness, sensitivity, and performance.
Continue reading: The Role of Graphene-Based Nanomaterials in Biosensors.
Reference
Wu, J. et al., (2021) Single-atom Au catalyst loaded on CeO2: A novel single-atom nanozyme electrochemical H2O2 sensor. Talanta Open, 4. 100075. Available at: https://doi.org/10.1016/j.talo.2021.100075
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.