Novel research in the journal Frontiers in Materials has explored the synthesis of multifunctional NiVCe-layered double hydroxide (NiVCe-LDH) nanoparticles. The NiVCe LDH nanomaterials demonstrated good selective charge and rate efficiency, indicating their great potential as an electrocatalyst for blended energy storage systems.

Study: NiVCe-Layered Double Hydroxide as Multifunctional Nanomaterials for Energy and Sensor Applications. Image Credit: cybrain/Shutterstock.com
NiVCe-LDH nanoparticles were revealed to have significant potential as electrode material in hybrid energy storage systems, oxygen evolution reactions (OER), and sensing applications.
Utilizing a quick and easy bulk infusion analysis methodology, trimetallic NiVCe-LDH-based screen-printed electrodes were designed for effectively detecting hydrogen peroxide in samples, reaching an acceptable recovery level of roughly 98 %.
Importance and Utilization of Layered Double Hydroxides
LDHs containing metals have developed as multi-purpose inorganic nanoparticles with substantial surface area, outstanding mechanical characteristics, and variable thermal and electric conductance.
Due to their intrinsic capabilities, LDH-based nanostructures have emerged as potential contenders as a solution for various technical and sustainability issues. These nanostructures are ideal for devices aimed at electrochemical conversion reactions as well as energy storage systems.
Importance and Utilization of Multifunctional Nanoparticles
Compared to traditional nanostructures, multi-purpose nanoparticles have greater potential to achieve objectives in a mutually beneficial manner, such as co-delivery of numerous bioactive(s) with image processing substances, destination-specific delivery via exterior receptor decoration, and concurrently achieving cancer therapeutic approaches and diagnostic tools.
Their capacity to integrate several characteristics, such as electrical, electromagnetic, photonic, and enzymatic, ensures the popularity of multi-purpose nanoparticles.
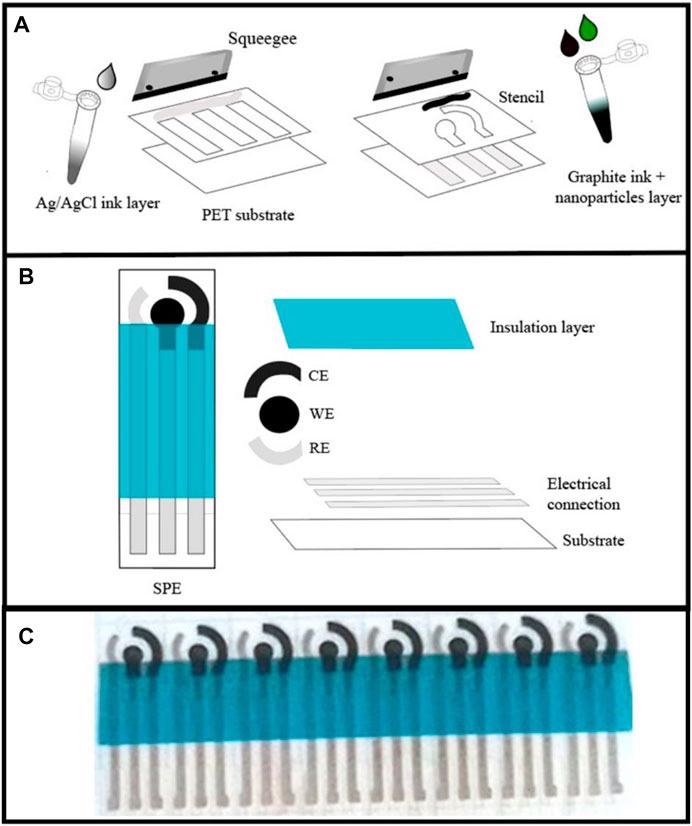
(A) Fabrication of screen-printed electrode and its coating with graphite ink loaded with 25% (in weight) of Ni0.9Ce0.05V0.05-LDH NPs. (B) A design drawing and (C) photography of electrochemical analytical devices with three-electrodes developed using screen printing. Image Credit: Gonçalves, J. M. et al.
The Sol-Gel Method of Synthesis
One professionally utilized technology is the sol-gel technique, which can create coverings on glass windows. Pristine ingredients are used as the starting material, enabling the procedure to positively influence the final product purity and concentration.
However, certain limitations exist, including the high concentration of pure substances utilized for the process, large material wastage, crack formations, and substantial volume shrinkages.
The latest research integrated Ce3+ and V5+ ions in the Ni(OH)2 composite. This resulted in the formation of a cost-effective nanocrystalline and multi-purpose trimetallic NiVCe-based hydroxide as a positively charged electrode. Applications of this nanomaterial include HSCs, enzymatic degradation for OER, and hydrogen peroxide quantitative determination.
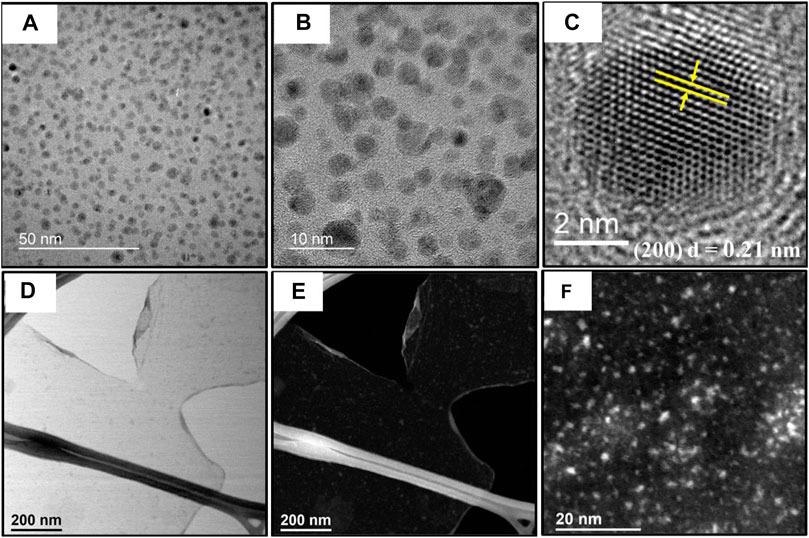
(A, B) TEM images of Ni0.9V0.05Ce0.05-LDH NPs/GO with magnification growing from left to right. (C) HRTEM of trimetallic oxide NPs formed in situ after dehydroxylation/dehydration reaction induced by electron beam. (D) Bright field-STEM image showing Ni0.9V0.05Ce0.05-LDH NPs dispersed on GO surface and (E) same image in (D) in HAADF mode. (F) Higher magnification HAADF-STEM image of Ni0.9V0.05Ce0.05-LDH NPs. Image Credit: Gonçalves, J. M. et al.
Research Results
The substance Ni0.9 V0.05 Ce0.05-LDH was prepared and analyzed. Basal reflections were observed, which are substantial proof of the presence of LDH structure.
The appearance of reflections patterns in HRTEM pictures also supports the existence of NPs with a significant degree of polymerization.
STEM pictures contained even smaller NPs on graphene oxide (GO), demonstrating that localized thermal treatment can also cause nanoparticles convergence.
CV was used to assess the electrochemical behavior of Ni0.9V0.05Ce0.05-LDH, and were measured between 0.0 and +0.6 V. The CVs showed a pair of waves in a suitable region for all probes. During the 100 redox sessions, the Ni0.9V0.05Ce0.05-LDH showed a steady and minor dynamic capability.
The Ni0.9V0.05Ce0.05-LDH/FTO electrode demonstrated a rise in particular power up to the 100th cycle, retaining around 55% of the specific charge at the conclusion of 1,000 cycles, identical to the CV data.
To test the evidence of the produced NPs' electrocatalytic capability, linear sweep voltammetry (LSV) studies in the solution of electrolyte were performed using a typical three-electrode setup. The Tafel slope of the Ni0.9V0.05Ce0.05-LDH electrocatalyst was 47 mV dec-1, while that of the -Ni(OH)2 was 46 mV dec-1. These results suggest that both electrode materials are viable for OER.
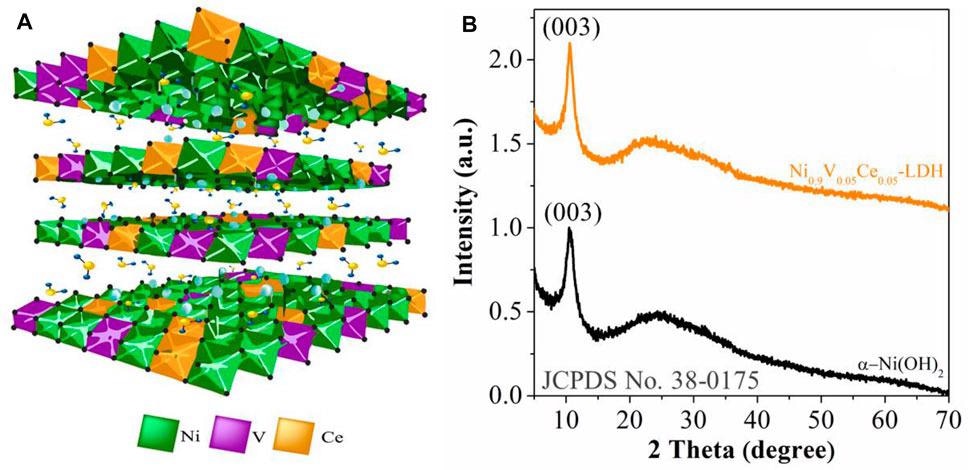
(A) Scheme depicting lateral view of Ni0.9V0.05Ce0.05-LDH. (B) X-ray diffractograms of α-Ni(OH)2 and Ni0.9V0.05Ce0.05-LDH NPs deposited on a glass slip substrate. Image Credit: Gonçalves, J. M. et al.
Importance of the Study
The fabrication of nanomaterials with multi-purpose layered double hydroxide comprising Ni, V, and Ce ions (Ni1-x-yVxCey-LDH, where x =y= 0.05) is described for the first time in this work. The lack of segmentation and the development of a homogenous trimetallic hydroxide composed of super-small (nanoparticles with diameters ranging from 2–3 nm) nanomaterials were observed.
The trimetallic Ni0.9V0.05Ce0.05-LDH investigated in the research paper showed outstanding prospects as multi-purpose electrode materials for sustainable technologies and for building devices with promising prospects for fluorometric usage.
Continue reading: What Does the Future Look Like for Graphene Supercapacitors?
Reference
Gonçalves, J. M. et al., (2021) NiVCe-Layered Double Hydroxide as Multifunctional Nanomaterials for Energy and Sensor Applications. Frontiers in Materials. 781900. Available at: https://www.frontiersin.org/articles/10.3389/fmats.2021.781900/full
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.