Reviewed by Mila PereraSep 21 2022
Investigation into the production of new materials could pave the way to more sustainable and eco-friendly products like light-emitting diodes (LEDs) and solar panels.
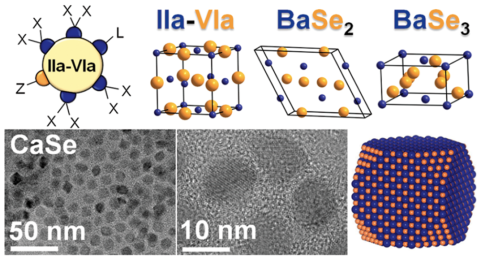 Unit cells and electron micrographs of alkaline earth chalcogenide (AeCh) nanocrystals. Image Credit: Ames National Laboratory
Unit cells and electron micrographs of alkaline earth chalcogenide (AeCh) nanocrystals. Image Credit: Ames National Laboratory
Researchers from Ames National Laboratory and Iowa State University have formulated a colloidal synthesis technique for alkaline earth chalcogenides. This technique enables them to regulate the size of the nanocrystals in a material. They also managed to examine the surface composition of the nanocrystals and evaluate the purity and optical features of the materials involved.
Researchers are increasingly interested in alkaline earth chalcogenides, a kind of semiconductor with various potential applications such as LEDs, bioimaging, and thermal sensors. Alkaline earth chalcogenides could also be employed to produce optical materials like perovskites, which turn light into energy.
According to Javier Vela, the John D. Corbett Professor of Chemistry at Iowa State University and Ames Lab scientist, one of the reasons for these new materials to be gaining interest is because, “they are comprised of earth-abundant and biocompatible elements, which make them favorable alternatives compared to the more widely used toxic or expensive semiconductors.”
Vela elucidated that more extensively employed semiconductors comprise cadmium or lead, both elements that are harmful to both human health and the environment. Furthermore, the most prevalent method researchers use to create these materials requires solid-state reactions.
These reactions often occur at extremely high temperatures (above 900 °C or 1652 °F) and require reaction times that can last anywhere from days to weeks.
Javier Vela, Scientist, Ames National Laboratory
On the other hand, Vela explained that “solution-phase (colloidal) chemistry can be performed using much lower (below 300 °C or 572 °F) temperatures and shorter reactions times.” The colloidal technique Vela’s team employed needs less time and energy to create the materials.
Vela’s team learned that the colloidal synthesis technique enabled them to regulate the nanocrystals’ size. This is vital as it establishes the optical features of certain materials. Vela showed that by altering the size of the particles, researchers can impact how well the materials capture light.
This means we can potentially synthesize materials that are more suited for specific applications just by changing the nanocrystal size.
Javier Vela, Scientist, Ames National Laboratory
Vela states that the team’s primary goal was to create semiconducting alkaline-earth chalcogenide perovskites because of their possible application in solar equipment. They required, however, a deeper insight into the fundamental chemistry of alkaline earth chalcogenides to achieve this goal. They therefore decided to concentrate on these binary materials instead.
Vela stated that their study fills a requirement to increase scientists’ comprehension of luminescent, photovoltaic, and thermoelectric materials that are composed of earth-rich and non-toxic elements.
We hope that our developments with this project ultimately aid in the synthesis of more complex nanomaterials, such as the alkaline-earth chalcogenide perovskites.
Javier Vela, Scientist, Ames National Laboratory
Journal Reference
Roth, A. N., et al. (2022) Alkaline-Earth Chalcogenide Nanocrystals: Solution-Phase Synthesis, Surface Chemistry, and Stability. ACS Nano. doi.org/10.1021/acsnano.2c02116.