Materials scientists are particularly interested in nanoparticles because of their extraordinary physical and chemical characteristics. Nanoparticles can be as small as one nanometer, or one billionth of a meter. They can only be viewed with a highly specialized electron microscope and are invisible to the unaided eye.
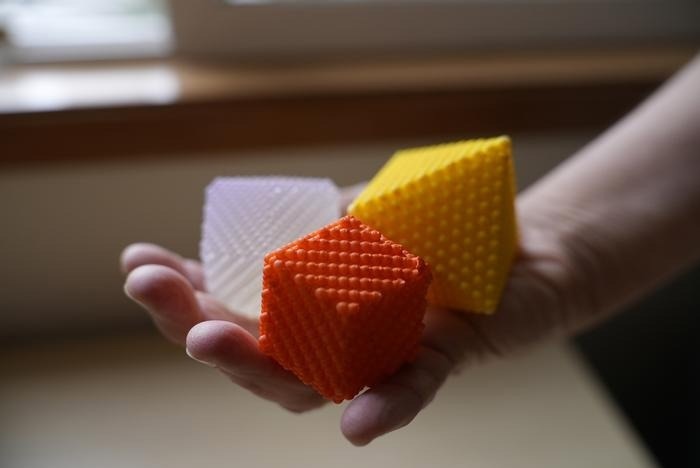 Anne Bentley, associate professor of chemistry at Lewis & Clark College in Portland, Oregon, has developed an innovative way to teach nanoscience, using 3D-printed models that make the unseen visible. Image Credit: Stephen Mercier/Lewis & Clark College
Anne Bentley, associate professor of chemistry at Lewis & Clark College in Portland, Oregon, has developed an innovative way to teach nanoscience, using 3D-printed models that make the unseen visible. Image Credit: Stephen Mercier/Lewis & Clark College
According to Anne Bentley, a Professor in the Chemistry Department at Portland, Oregon’s Lewis & Clark College, the subject of nanoscience was made feasible by developments in imaging technology in the 1990s and early 2000s.
I think a lot of chemistry is outside the realm of what people can hold in their hands. You can obtain evidence about what’s going on, but you’re still investigating something that’s at too small a scale for your eyes to see. Anything you can do to scale it up is helpful.
Anne Bentley, Associate Professor, Chemistry, Lewis & Clark College
Bentley produced three-dimensional representations of the most basic geometric forms that nanoparticles can take on. She co-authored a study titled “A Primer on Lattice Planes, Crystal Facets, and Nanoparticle Shape Control,” which was published in the Journal of Chemical Education.
She has made the directions for making these models available, which can be constructed using paper or 3D printing material.
A Primer for Materials Chemistry Students
Nanoparticles are made of atoms organized in a three-dimensional pattern and can take on a variety of geometric patterns. Like the cuts of a gemstone, the shapes have flat surfaces known as planes or facets. According to Bentley, the configuration of atoms on these crystal surfaces affects the unique qualities of the material.
Bentley added, “The shapes are derived from this packing of the atoms. The motivation to make different shapes really comes down to the arrangement of the atoms when the material is sliced in different ways on different crystal planes.”
Bentley identifies low-index shapes as the three easiest ways to slice a structure, and this is the emphasis of her studies.
“There are lots more complex ways to slice it, but these are the three fundamental ways to do it, by making them either six, eight, or twelve sides―cubes, octahedra, or rhombic dodecahedra. It was a natural choice to focus on those three for the article,” Bentley stated.
Transforming a “Jumble of Numbers” into Shapes
Bentley further stated, “Nanoscience is a topic that both falls between chemistry and physics in the curriculum, but also between undergraduate- and graduate-level research. It is important that beginning materials chemists have a fundamental understanding of crystal planes, facets, and directions of growth. They also need to understand the three-digit notation system used to index these attributes, known as the Miller indices. Otherwise, this system can look like a mysterious jumble of numbers.”
She believed it was essential to offer a foundation of information in a way that was accessible so that teachers could help raise awareness of this significant and expanding topic. Bentley thinks there are benefits to being able to hold the models in your hands, even if more intricate structures than the 3D-printed ones can be produced digitally using computer simulation tools.
She added, “I like things I can look at and think about.”
She went on to say that 3D models are extremely valuable for developing a grasp of this important nanoscience topic.
Growing Gold Particles to Convert Carbon Dioxide
Bentley and her students work at Bentley’s lab, manipulating gold atoms in liquid vials to alter the forms of nanoparticles.
She added, “You need to just make the right conditions at the right temperatures, a whole environment that is conducive to growing a particular shape.”
Bentley is interested in gold nanoparticles because of their catalytic capabilities or capacity to speed up chemical processes. She stated that the way the material is sliced exposes distinct atom patterns. Previous study has found that a certain form of gold nanoparticle, the 12-sided rhombic dodecahedra, is more successful at converting carbon dioxide into fuel materials.
Bentley concluded, “It is like recycling. Not only does this nanoparticle shape enable researchers to remove carbon dioxide from the atmosphere, but it allows them to turn it back into some kind of fuel that can be used. So if we can grow particles that have this facet on them only, that’s a real advantage.”
Journal Reference:
Bentley, A. K, et. al. (2023) A Primer on Lattice Planes, Crystal Facets, and Nanoparticle Shape Control. Journal of Chemical Education. doi:10.1021/acs.jchemed.3c00371.