Numerous innovative chemical systems for photoinduced electron transfer have been presented by scientists throughout the years. Japanese scientists have already made progress in this direction by creating a copolymer-conjugated nanocatalytic device that effectively promotes photoinduced electron transport.
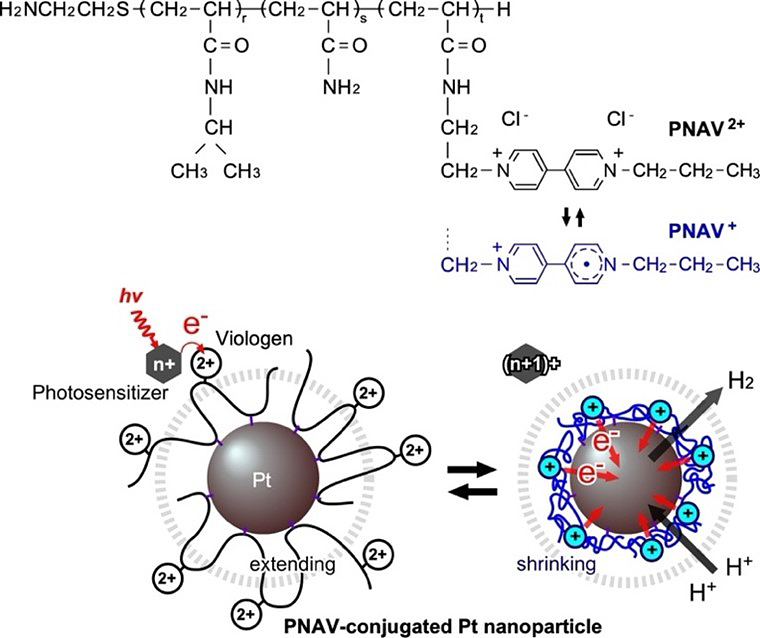 Photoinduced hydrogen generation system employing poly(NIPAAm-co-AAm-co-Viologen) (PNAV). The chemical structure and redox states of PNAV, and the electron transfer driven by coil-globule transitions of PNAV conjugated on Pt nanoparticle (NP). The dotted circle represents the effective distance from the Pt NP surface. Image Credit: Kosuke Okeyoshi from JAIST
Photoinduced hydrogen generation system employing poly(NIPAAm-co-AAm-co-Viologen) (PNAV). The chemical structure and redox states of PNAV, and the electron transfer driven by coil-globule transitions of PNAV conjugated on Pt nanoparticle (NP). The dotted circle represents the effective distance from the Pt NP surface. Image Credit: Kosuke Okeyoshi from JAIST
The team connected platinum nanoparticles to a temperature-responsive ternary random copolymer. This invention can have a significant impact on electrochemical processes, bioinspired soft materials, artificial photosynthesis, macromolecular identification, and dynamic electron transfer by promoting photoinduced hydrogen creation.
For photoinduced, or light-driven, electron transfer, researchers have created a variety of molecular systems, such as supramolecules, hybrid materials, and organic polymeric systems.
Although these systems meet the distance requirement set by the acceptor and donor for effective electron transfer, they are often not able to accommodate molecule mobility, particularly in fluid conditions.
A new study has explored whether a system that promotes electron movement with the above constraints is possible.
A group of scientists from the Japan Advanced Institute of Science and Technology (JAIST), led by Associate Professor Shun Nishimura, Graduate course student Reina Hagiwara, and Associate Professor Kosuke Okeyoshi, have created a copolymer-conjugated nanocatalytic system to improve active electron transfer for more photoinduced hydrogen generation.
Their study, which was published in Chemical Communications, attempts to address the shortcomings of the systems that currently use photoinduced electron transfer. The study aimed to develop a catalyst system that could effectively facilitate electron transfer while causing the fewest possible side effects.
This system has potential real-life applications for the hydrogen economy. By integrating the system with an oxygen-generating system, photoinduced water splitting (artificial photosynthesis) is anticipated.
Kosuke Okeyoshi, Associate Professor, Japan Advanced Institute of Science and Technology
One well-known molecule that is an effective electron giver and acceptor is Viologen. This characteristic of Viologen was previously used by researchers to create an electron transfer system that includes modified platinum nanoparticles (Pt NPs) and the copolymer poly(N-isopropylacrylamide-co-Viologen) (PNV).
In this system, Viologen’s redox changes trigger a temperature-dependent phase shift in PNV, which enables a cyclical electron transfer mechanism for continuous hydrogen production. Even then, free PNV molecules located further away were also capable of accepting electrons, even though the PNVs close to the Pt NPs took part in the electron transfer process.
Utilizing the ternary random copolymer poly(NIPAAm-co-Acrylamide-co-Viologen), or PNAV, which was created by carefully regulating the molecular weight and introduction ratio of the polymeric units, the researchers have now developed a copolymer-conjugated nanocatalytic system to solve this problem.
One of the most remarkable features of PNAV is its behavior in response to temperature, which is indicated by a temperature-dependent phase transition. In its reduced form (PNAV+), this special copolymer displays a noticeable shift, fluctuating between a shrunken state and a bloated state in its oxidized form (PNAV2+).
Furthermore, a reduction technique is used to attach PNAV to Pt NPs, giving control over the separation between the viologen and the Pt NPs. More specifically, the effectiveness of the suggested cyclic electron transfer mechanism at a given distance depends on the exact swelling/shrinking of PNAV on the Pt NPs.
The current invention uses a stimuli-responsive polymer chain's advantages to accomplish dynamic electron transfer. In addition to showing promise for enabling active electron transfer in photoinduced hydrogen production, the copolymer-conjugated nanocatalytic system could also be useful in artificial photosynthetic processes like photoinduced water splitting.
Furthermore, it is expected that this novel method will find wider uses in other fields outside photochemical processes, such as electrochemical reactions and macromolecular identification.
Thus, this technology’s ability to provide persistent cyclic electron transfer offers prospects for breakthroughs in a variety of scientific fields.
Okeyoshi concluded, “The long-term implications include the promotion of a hydrogen energy society powered by sunlight and the manufacturing of bio-inspired soft materials as products.”
Grant-in-Aid for Challenging Research (Exploratory) (Grant number: JP21K18998) from The Ministry of Education, Culture, Sports, Science and Technology (MEXT) funded the study.
Journal Reference:
Hagiwara, R., et. al. (2023) Precise design of copolymer-conjugated nanocatalysts for active electron transfer. Chemical Communications. doi:10.1039/D3CC05242G