Dr. Iulian Gherasoiu, an associate professor of electrical and computer engineering technology at SUNY Polytechnic Institute (SUNY Poly), and colleagues published a study in the Journal of Applied Electrochemistry that uncovered the characteristics of inexpensive MoVN/MoNi4-MoO2 nanorods made by a simple, two-step hydrothermal process.
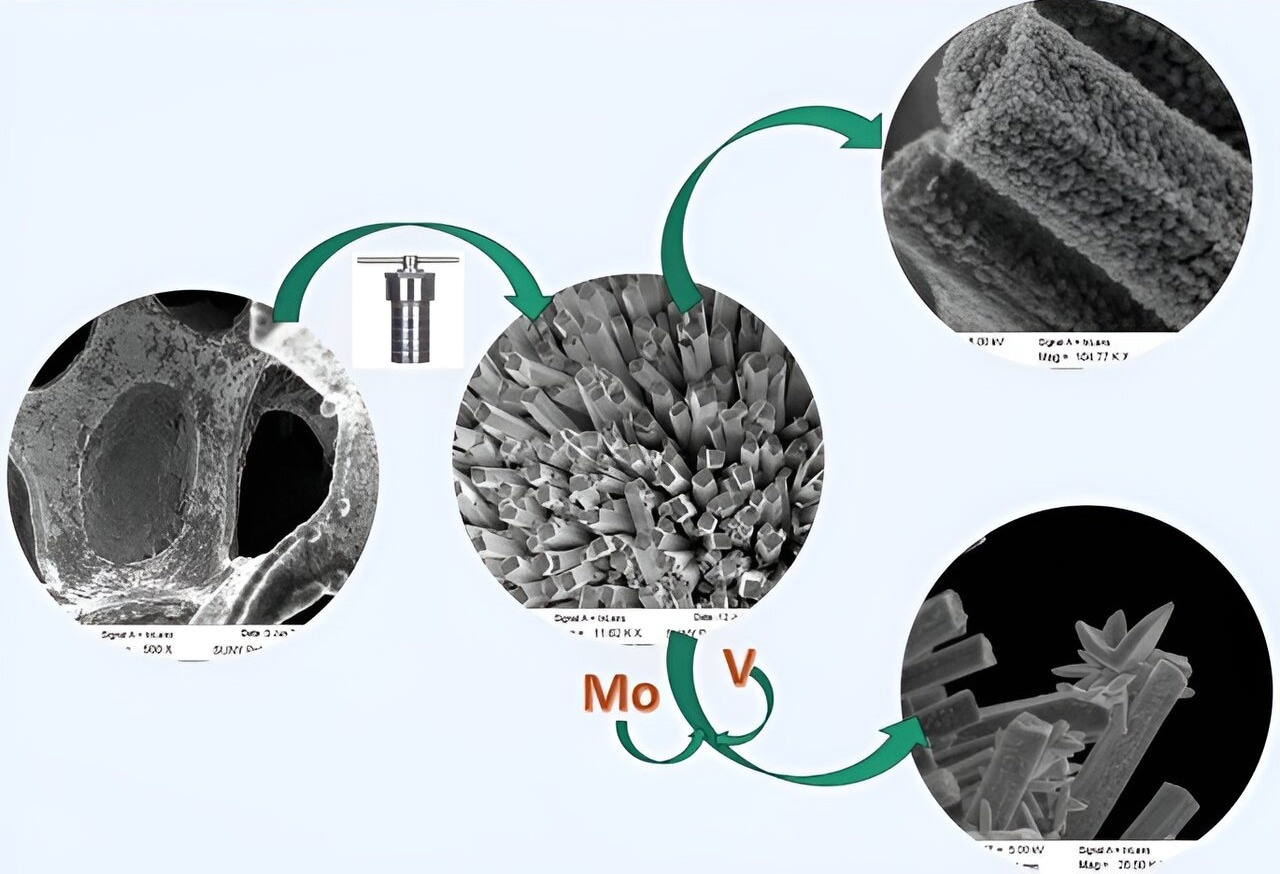 Graphical abstract. Image Credit: Journal of Applied Electrochemistry (2024). DOI: 10.1007/s10800-023-02064-x
Graphical abstract. Image Credit: Journal of Applied Electrochemistry (2024). DOI: 10.1007/s10800-023-02064-x
According to Gherasoiu, the investigation of practical methods for producing molecular hydrogen is motivated by the growing demand for clean and renewable energy. Water electrolysis is a viable option for producing pure hydrogen with outstanding efficacy when supported by highly active, non-noble metal electrocatalysts.
However, this reaction occurs almost entirely on Pt/C catalysts at the cathode, which are costly and must be replaced with a metal-based catalyst with similar HER (hydrogen evolution reaction) activity.
The electrodes performed exceptionally well, with a high specific electrochemical surface area, low overpotential for both half-cell reactions (HER and OER) and negligible degradation, paving the way for the development of low-cost and highly effective electrodes as a possible substitute for Pt-based electrodes for use in commercial electrolyzers.
Journal Reference:
Kumaran, Y., et. al. (2024) MoVN-coated MoNi4-MoO2 nanorods as a bifunctional electrode for electrochemical water splitting. Journal of Applied Electrochemistry. doi:10.1007/s10800-023-02064-x