May 7 2009
Researchers at Sandia National Laboratories have successfully designed and demonstrated key features of a hydrogen storage system that utilizes a complex metal hydride material known as sodium alanate. The system, developed through a multiyear project funded by General Motors Corp., stores 3 kilograms of hydrogen and is large enough to evaluate control strategies suitable for use in vehicle applications.
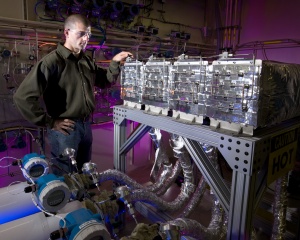 Sandia engineer Terry Johnson surveys various components of the hydrogen storage system he and his team designed for General Motors. To the right is the "SmartBed," featuring a thermal management system with individual control of four identical modules, each of which is a shell and tube heat exchanger. The material used to store the hydrogen – sodium alanate – resides within the tubes. (Photo by Randy Wong)
Sandia engineer Terry Johnson surveys various components of the hydrogen storage system he and his team designed for General Motors. To the right is the "SmartBed," featuring a thermal management system with individual control of four identical modules, each of which is a shell and tube heat exchanger. The material used to store the hydrogen – sodium alanate – resides within the tubes. (Photo by Randy Wong)
The design tools developed by Sandia researchers now provide GM with a workable template for future designs, which is expected to significantly save the company costs and time when developing hydrogen storage systems for onboard vehicular applications.
“For GM, the enduring value of this project can be found in the design concepts, computational tools, and control strategies that Sandia developed,” said Jim Spearot, GM lead executive for hydrogen storage. “With this new body of knowledge and information, we will be able to quickly design viable systems as new storage materials emerge.”
Methods have been validated
Sandia researchers are quick to point out that the system was not meant to fit on board a vehicle, and that sodium alanate will not be the material of choice for onboard storage of hydrogen. But, although it is indeed larger and heavier than a viable automotive storage system requires, the system’s engineered elements address many of the thermal management issues that are necessary for successful vehicular storage of hydrogen.
“We’ve shown that we can engineer vehicle-scale energy storage systems to meet a variety of operating requirements and driving cycles, and our design methods have been validated for relevant materials,” said Sandia engineer Terry Johnson.
Johnson said Sandia is well-equipped to do similar work on behalf of other companies, including those that manufacture rolling stock, specialty, or heavy-duty vehicles. Companies that focus on other niche applications, including underwater, military, or unmanned aerial vehicles, would likely benefit from Sandia’s expertise, too, he said.
Modular heat exchange system allows flexibility
In addition to its size and storage capacity, the unique features of the Sandia system include an advanced heating system whereby a fraction of the stored hydrogen is used to provide heat to release the remaining hydrogen. This method — the catalytic combustion of hydrogen — is not new, Johnson said, but is unique to this particular application and the first to be successfully demonstrated. “We chose not to use resistive (electrically driven) heating, because it would have necessarily resulted in a larger and heavier system,” he said.
After considering a number of thermal management options, Sandia selected a “shell and tube” heat exchanger, a heating technique common in many industrial processes. The “SmartBed” — a term coined by Sandia that refers to the method for controlling a modular storage system — consists of four identical modules, each of which contains a shell and tube heat exchanger. The material used to store the hydrogen — sodium alanate — resides within the tubes, which essentially serve as a high-pressure storage vessel. Inside the shell, a heating fluid circulates to transfer heat to and from the sodium alanate.
The modular design of the system means that only a minimum amount of the storage material needs to be heated at any one time. The design also aids in the packaging of the system to fit on board a vehicle.
Sandia’s work with GM on a hydrogen storage system reflects the lab’s long history of exploring basic science for energy and transportation. From developing renewable means of producing hydrogen, to discovering the science behind hydrogen safety, to creating the building blocks of hydrogen and fuel cell systems, Sandia scientists and engineers are actively working to help hydrogen and fuel cells take their place in a sustainable energy future. Sandia is actively seeking commercial partners to further develop its hydrogen storage technologies.