Mar 6 2013
New research from Rice University, Baylor College of Medicine (BCM) and the Puget Sound Blood Center (PSBC) has revealed how stresses of flow in the small blood vessels of the heart and brain could cause a common protein to change shape and form dangerous blood clots. The scientists were surprised to find that the proteins could remain in the dangerous, clot-initiating shape for up to five hours before returning to their normal, healthy shape.
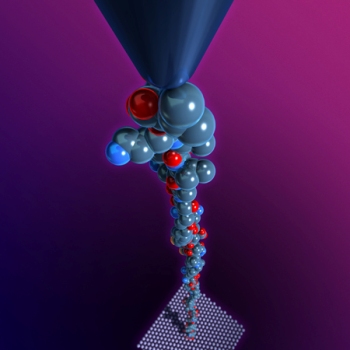 Rice University researchers in the lab of Ching-Hwa Kiang use the bobbing needle from an atomic force microscope to grab and pull individual protein molecules. By stretching the proteins, Kiang's team can measure the precise physical forces that shape them. CREDIT: C. Kiang/Rice University
Rice University researchers in the lab of Ching-Hwa Kiang use the bobbing needle from an atomic force microscope to grab and pull individual protein molecules. By stretching the proteins, Kiang's team can measure the precise physical forces that shape them. CREDIT: C. Kiang/Rice University
The study — the first of its kind — focused on a protein called von Willebrand factor, or VWF, a key player in clot formation. A team led by Rice physicist Ching-Hwa Kiang found that “shear” forces, like those found in small arteries of patients with atherosclerosis, cause snippets of nonclotting VWF to change into a clot-forming shape for hours at a time. The finding appears online this week in Physical Review Letters.
“When I first heard what Dr. Kiang’s team had found, I was shocked,” said blood platelet expert Dr. Joel Moake, a study co-author who holds joint appointments at Rice and BCM. Moake, whose research group was the first to describe how high shear stress could cause platelets to stick to VWF, said, “I had thought that the condition might last for such a short time that it would be unmeasurable. No one expected to find that this condition would persist for hours. This has profound clinical implications.”
Kiang, associate professor of physics and astronomy and of bioengineering, studies the forces involved in protein folding. Proteins are the workhorses of biology. Tens of thousands are produced each second in every living cell, and each of these folds into a characteristic shape within moments of its creation. Despite its ubiquity, protein folding is an immensely complex process that is shrouded in mystery.
Kiang is a pioneer in the use of atomic force microscopes (AFM) to shed light on the fundamental physical processes involved in protein folding. The AFM has a tiny needle with a tip measuring just a few atoms across. The needle is suspended from a tiny arm that bobs up and down over a surface. Kiang’s team uses the bobbing needle to grab and pull apart individual protein molecules. By stretching these like rubber bands, her team has shown it can measure the precise physical forces that hold them in their folded shape.
“In this study, we did more than just measure the forces; we used those measurements to see what state the molecule was in,” Kiang said. “In this way, we were able to study the dynamics of the molecule, to see how it changed over a period of time.”
Moake, a senior research scientist in bioengineering at Rice and professor of medicine at BCM, said the work is vitally important because it helps explain the workings of VWF.
“VWF is synthesized in the cells that line the walls of blood vessels, and it’s stored there until the cells get signals that the vessels are in danger of injury,” Moake said. “In response to those stimuli, the cell secretes VWF. It’s a long protein, and one end remains anchored to the cell while the rest unfurls from the wall like a streamer.”
The act of unfurling makes VWF sticky for platelets, and that begins the process of hemostasis, which prevents people from bleeding to death when blood vessels are damaged by cuts and wounds.
“The body recognizes when clotting must stop — when there are too many strings, too much sticking, too many platelet clumps — and it uses an enzyme to clip the long VWF strings,” Moake explained. “First, it makes large, soluble versions of the strings that remain somewhat sticky, and then these large soluble portions of VWF are reduced into smaller subunits of VWF that circulate in the plasma.”
Under normal conditions, these circulating subunits, which are called PVWF, fold into compact shapes and cease to be sticky to platelets. However, previous research had shown that a type of physical stress called “shear” — which can arise in partially occluded arterial blood vessels with high flow rates — could cause PVWF to become sticky to platelets.
“That’s all we knew,” Moake said. “We didn’t know how the conformation of the PVWF protein changed. That is why Dr. Kiang’s research is so important and makes it more likely that therapeutic interventions can be more rationally designed.”
To study the problem, Kiang’s lab worked closely with Moake’s team at Rice’s BioScience Research Collaborative and with researchers from the laboratory of co-author Jing-fei Dong, formerly of BCM and now at PSBC in Seattle. Moake’s and Dong’s groups prepared samples of PVWF, subjecting some to the shear stresses known to induce clot formation. Kiang’s team used AFMs to test the samples. Through a combination of experiments and deductive reasoning, her team determined exactly which portion of PVWF changed its conformation during shear stress. They also determined how long the protein remained partially unfurled before relaxing into its natural shape.
“The next step will be to design new experiments that allow us to monitor the proteins as they bind to platelets and initiate clot formation,” Kiang said. “That will tell us even more about the physical properties of the proteins and provide more clues about potential therapies.”
The research was supported by the National Institutes of Health, the National Science Foundation, the Alliance for NanoHealth, the Welch Foundation, the Mary R. Gibson Foundation and the Everett Hinkson Fund. Study co-authors include Rice graduate students Sithara Wijeratne and Eric Frey, former Rice graduate student Eric Botello, BCM researchers Hui-Chun Yeh and Angela Bergeron, Rice undergraduate Jay Patel, PSBC’s Zhou Zhou and Rice senior research technicians Leticia Nolasco and Nancy Turner.