Jan 28 2019
The noble metal—platinum—is oxidized more rapidly than expected under technologically relevant conditions. This finding was revealed from a study jointly performed by the University of Vienna and the DESY NanoLab.
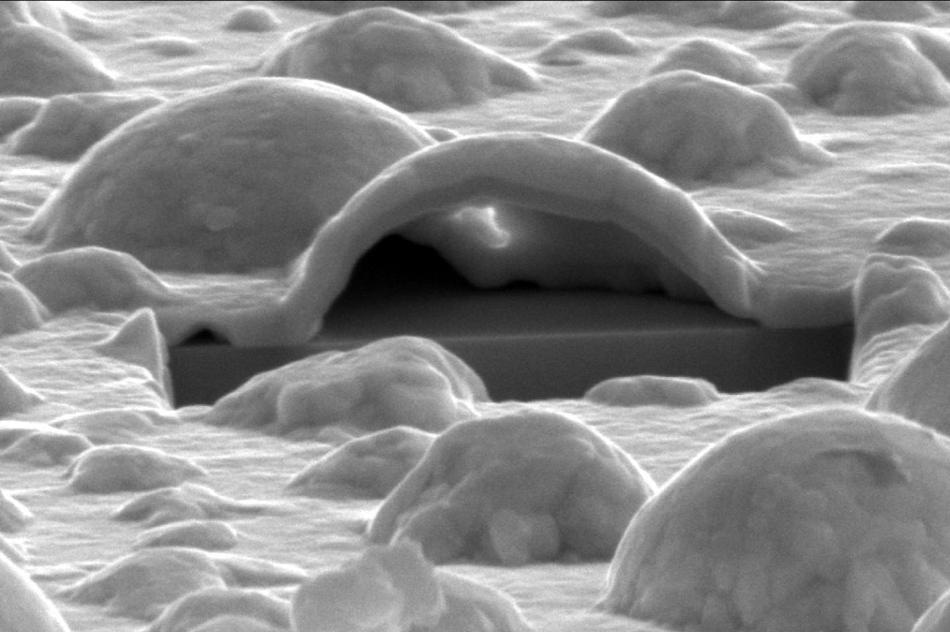 This is an electron microscope view into the interior of a platinum bubble. The cross-section was exposed with a focused ion beam. Below the hollow Pt bubble the angular YSZ crystal can be seen. (Image credit: DESY, Satishkumar Kulkarni)
This is an electron microscope view into the interior of a platinum bubble. The cross-section was exposed with a focused ion beam. Below the hollow Pt bubble the angular YSZ crystal can be seen. (Image credit: DESY, Satishkumar Kulkarni)
Devices containing platinum, like the catalytic converters used for reducing exhaust emissions in vehicles, can have reduced efficiency because of this reaction. The research team, headed by Thomas Keller, the principal author from DESY and the University of Hamburg, has reported its findings in the journal Solid State Ionics.
Platinum is an extremely important material in technological terms. The conditions under which platinum undergoes oxidation have not yet been fully established. Examining those conditions is important for a large number of applications.
Thomas Keller, Study Principal Author, DESY NanoLab and the University of Hamburg
A thin platinum layer, which had been applied to an yttria-stabilized zirconia crystal, or YSZ crystal, was examined by the researchers. That is the same kind of combination utilized in the lambda sensor integrated in automotive exhaust emission systems. The YSZ crystal is considered as an ion conductor, which means that it is capable of conducting electrically charged atoms (ions), in this example, oxygen ions. The platinum layer, deposited through vapor, acts as an electrode. After measuring the oxygen content of the exhaust fumes in the car, the lambda sensor changes this into an electrical signal; this signal electronically regulates the combustion process in order to reduce toxic exhausts.
The researchers at DESY NanoLab applied a potential difference of approximately 0.1 V to the platinum-coated YSZ crystal and subsequently heated it to about 450 °C—conditions akin to those found in various technical devices. Consequently, oxygen accumulated under the impermeable platinum film and reached pressures of around 10 bars, equivalent to the pressures in lorry’s tires.
The oxygen-exerted pressure, together with the increased temperature, resulted in the formation of tiny bubbles within the platinum film, usually having a diameter of roughly 1000 nm (0.001 mm).
Platinum blistering is a widespread phenomenon, and we would like to develop a better understanding of it. Our investigation can also be considered representative of this type of electrochemical phenomenon at a range of other boundary layers.
Thomas Keller, Study Principal Author, DESY NanoLab and the University of Hamburg
Using a so-called focused ion beam, or FIB, as a kind of ultra-sharp scalpel, the researchers sliced open the platinum bubbles and examined their content more closely. They ultimately discovered that the bubbles’ inner surface was lined with a platinum oxide layer, which could have a thickness of around 85 nm, relatively thicker than predicted.
“This massive oxidisation took place in conditions under which it is not normally observed,” reported co-author Sergey Volkov, who has penned his doctoral thesis at the University of Hamburg on this subject. “As a rule, platinum is a highly stable material, which is precisely why it is chosen for many applications, such as catalytic converters in cars, because it is not easily altered. Our observations are therefore important for such applications.”
The researchers believe that the high pressure of the oxygen inside the bubble might be responsible for accelerating the metal’s oxidation. This fact has to be taken into consideration in electrochemical sensor operation.