Mar 15 2019
The forthcoming energy crisis and huge energy demands of electronic devices have brought attention to the field of energy storage.
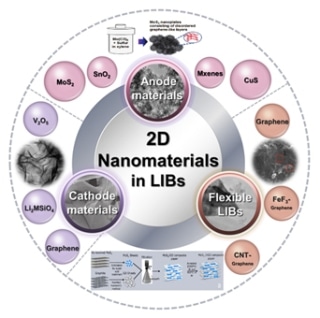 An overview illustration of the 2D nanomaterials with various structure and excellent performance utilized in lithium-ion batteries from three aspects of anode materials, cathode materials, and flexible batteries. (Image credit: World Scientific)
An overview illustration of the 2D nanomaterials with various structure and excellent performance utilized in lithium-ion batteries from three aspects of anode materials, cathode materials, and flexible batteries. (Image credit: World Scientific)
In a new study reported in the forthcoming issue in NANO, scientists from the China University of Petroleum (East China) have outlined the recent developments in the use of two-dimensional (2D) nanomaterials on the electrode materials of lithium-ion batteries, due to their extraordinary mechanical and electrochemical properties, making them excellent candidates as electrodes in high-capacity and long-cycle-life Li-ion batteries.
It has been observed that environmental pollution is becoming a very serious problem. Also, the tussle between energy crisis and increasing power demands of electronic devices is becoming very severe. So how to deal with such issues? It has been found that the application of high-performance energy storage devices, such as Li-ion batteries, is one of the successful approaches.
To achieve high capacity and long cycle life, many attempts have been made toward enhancing the electrochemical performance of the electrode materials. Recently, due to their fascinating electrochemical and mechanical properties, 2D nanomaterials have been driven to the forefront in the electrode material research.
2D nanomaterials possess sheet-like structures whose lateral size is >100 nm, but the thickness is just single or few atoms. The special structure has been endowed with its striking properties, like short diffusion distances, high-specific surface area, superior electrical conductivity, and electrochemical and thermal stability. Based on the composition, these materials can be divided into five types, namely, nonmetallic compound, element, metallic compound, salt, and organic compound. These are extremely required in many parts of Li-ion batteries (anodes and cathodes).
2D nanomaterials acting as anodes can yield high theoretical capacity. Graphene and graphene-based composite materials, including nonmetal/graphene, carbon nanotubes/graphene, sulfide/graphene, transition metal oxides/graphene, and salts/graphene, are the popular candidates. Moreover, there are other types of 2D nanomaterials with merits and demerits. For instance, MoS2 exhibits good capacity and less cycling stability and rate capacity. SnO2 has easy accessibility and low cost and toxicity, but the actual capacity is less than the theoretical capacity. MXene exhibits low diffusion barrier, good electrical conductivity, low open-circuit voltage, and high lithium capacity, but the manufacturing should be further examined to improve the surface functional groups.
2D nanomaterials acting as cathodes have a remarkably high theoretical capacity, electron transport velocity, and excellent structural stability. They are subdivided into four categories: (1) V2O5, with greater theoretical capacity; (2) graphene-related materials (graphene-modified LiFePO4, LiMn2O4, LiCoO2, and so on), which enhance cycling performance of the conventional cathode materials; (3) Li2MSiO4, with good thermal stability; (4) others (covalent organic frameworks), with excellent rechargeability. The 2D nanomaterials, with respect to their layered structure, can be easily constructed into flexible Li-ion batteries, particularly graphene and graphene-based composite materials. It complies with the development of portable electronic products.
Finally, the precise cathode and anode materials and their corresponding effects are outlined. Although there is a great demand, it is still a major challenge to improve the production rate and control the exact structure of 2D nanomaterials. This review has paved the way to show the recent research progress of 2D nanomaterials in Li-ion batteries, achieve the challenge, and forecast future investigations.
At present, the research team is analyzing the syntheses and assembly of nanomaterials and the use of nanomaterials in energy storage and environmental engineering.
Teng Wang, Zhiyuan Han, and Lingtong Li from China University of Petroleum (East China) and Xin Wu from Colorado School of Mines are other coauthors of this study.
This study was supported by the Fundamental Research Funds for the Central Universities (# 18CX02158A).