Researchers from the Institute of Physics of the Chinese Academy of Sciences, together with collaborators from the University of Science and Technology of China, have revealed the formation of boron clusters with magic numbers on monolayer borophene and observed the evolution process from monolayer borophene with adsorbed boron clusters to bilayer borophene.
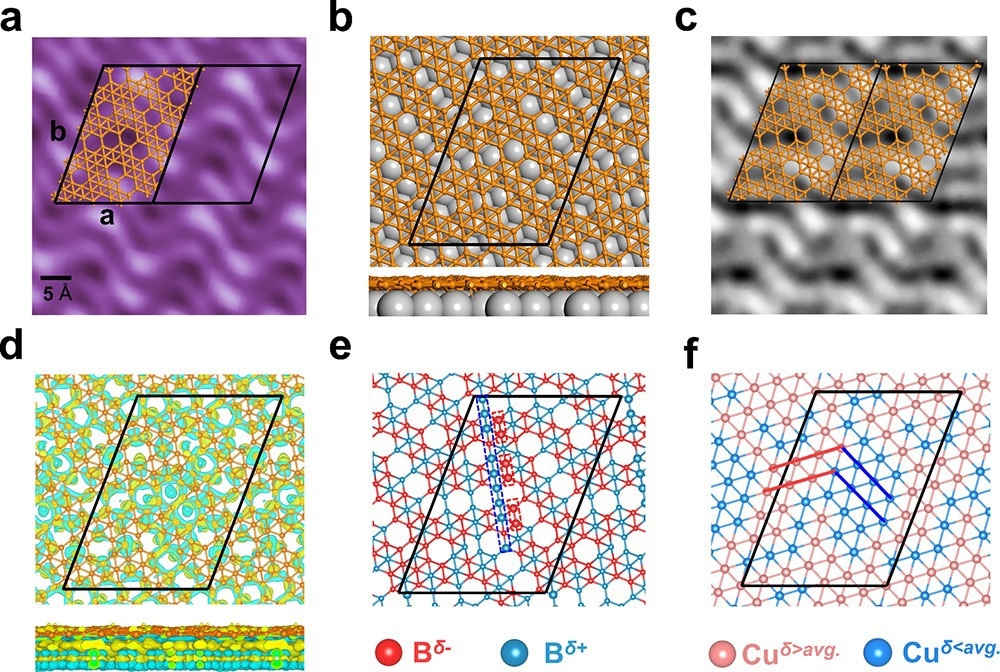 Fig. 1. The scanning tunneling microscope (STM) characterization, structural motifs, simulated STM image and charge redistribution of monolayer borophene on Cu(111). Image Credit: Institute of Physics
Fig. 1. The scanning tunneling microscope (STM) characterization, structural motifs, simulated STM image and charge redistribution of monolayer borophene on Cu(111). Image Credit: Institute of Physics
The study was published online in PNAS on Mar. 6.
Boron, which is next to carbon in the periodic table, has one electron less than carbon. This electron deficiency gives rise to its strong bonding ability and diverse boron polymorphs. Despite their variations, all boron polymorphs have as their building blocks B12 clusters, which have an icosahedral cage structure. However, research combining experimental and theoretical approaches has shown that small boron clusters in the gas phase, such as Bn (n = 3 to 12, 36), are planar or quasi-planar. This property has opened up the possibility of two-dimensional (2D) boron, also known as borophene.
According to the polymorphic nature of borophene, the growth of boron clusters on metal surfaces can strongly influence its structure. However, previous studies have mainly focused on small boron clusters in the gas phase, and only a few theoretical studies have investigated the evolution of boron clusters from small clusters to 2D sheets on surfaces.
In this study, the researchers discovered that, the growth of boron atoms onto a Cu(111) surface covered with a monolayer of borophene could generate boron clusters with a unique size distribution. Each boron cluster binds selectively to specific sites in each unit cell of the borophene monolayer, resulting in periodic arrays.
Density functional theory (DFT) calculations identified the chemically adsorbed boron clusters as B5 clusters, and the formation and arrangement of these clusters on the surface were found to be the result of the in-plane charge distribution and electron delocalization in the monolayer borophene.
In addition, the dense adsorption of B5 clusters on the monolayer borophene was observed to lead to spontaneous transformation into bilayer borophene.
This study was supported by the National Science Foundation of China, the Ministry of Science and Technology of China, the Chinese Academy of Sciences and the Beijing Natural Science Foundation.