Reviewed by Alex SmithJul 19 2022
In recent times, the transformation and utilization of renewable energy have become increasingly important for progress toward environmental and energy sustainability.
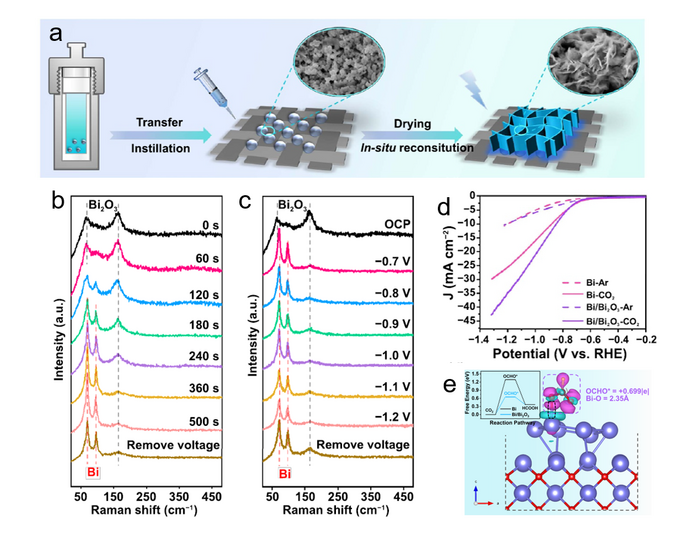 (a) Synthetic schematic diagram of Bi/Bi2O3. (b) Time-dependent Raman spectra at −1.01 V vs. RHE. (c) Potential-dependent Raman spectra in the CO2-saturated electrolyte. (d) Linear sweep voltammetry curve in 0.5 M NaHCO3 solution saturated with Ar/CO2 for CO2RR test. (e) DFT calculation result of Bi-based catalysts on CO2 reduction process. Image Credit: Hongbo Wang, Chongyang Tang, Bo Sun, Jiangchao Liu, Yan Xia, Wenqing Li, Changzhong Jiang, Dong He, Xiangheng Xiao.
(a) Synthetic schematic diagram of Bi/Bi2O3. (b) Time-dependent Raman spectra at −1.01 V vs. RHE. (c) Potential-dependent Raman spectra in the CO2-saturated electrolyte. (d) Linear sweep voltammetry curve in 0.5 M NaHCO3 solution saturated with Ar/CO2 for CO2RR test. (e) DFT calculation result of Bi-based catalysts on CO2 reduction process. Image Credit: Hongbo Wang, Chongyang Tang, Bo Sun, Jiangchao Liu, Yan Xia, Wenqing Li, Changzhong Jiang, Dong He, Xiangheng Xiao.
However, the use of occasional wind and solar energy will result in high electricity storage costs. Electrocatalytic CO2 reduction reaction (CO2RR) technology has attracted a lot of attention as it converts CO2 into high-value-added products for carbon neutralization and energy storage.
Scientists eventually realized that these metal compounds underwent dynamic structural evolution during the reduction process, which could result in the ambiguity of active species as the CO2RR research progressed.
More notably, external environmental factors severely limit the micromorphology and structure of the catalyst (it is affected by external potential, electrolytes, and other factors). There is a pressing need to use in-situ spectroscopic characterizations to monitor its true evolutionary process and aid in the establishment of a true active electronic structure model.
The preceding is extremely important for studying the structure-activity relationship and identifying the reaction active sites in the CO2RR system.
The research group, led by Professor Xiangheng Xiao of Wuhan University’s School of Physics and Technology, published their findings in a study in the International Journal of Extreme Manufacturing.
The surface reconstruction process of Bi2O3 nanoparticle catalysts in CO2 electroreduction was extensively investigated using in-situ Raman spectroscopy, as well as the internal reaction process.
In CO2 electroreduction, the surface reconstruction of the catalyst was first characterized using ex-situ techniques and in-situ Raman spectroscopy. The results revealed that the Bi2O3 nanoparticles were converted into two-dimensional thin-layer Bi/Bi2O3 nanosheets (4-6 nm). In the electrocatalytic process, it is regarded as the active phase.
The catalytic performance of the Bi/Bi2O3 nanosheets was good, with a Faraday efficiency (FE) of 94.8% for formate and a current density of 26 mA cm-2 at -1.01 V while maintaining a 90% FE over a broad possible range and long-term stability.
Theoretical calculations support the assertion that the enhanced performance is due to the improved bonding state of the surface Bi-Bi, which stabilized the adsorption of the key intermediate OCHO* and therefore elevated format production.
These researchers summarize the benefits and features:
“1. The evolution process of Bi2O3 nanoparticles to Bi/Bi2O3 nanosheets is successfully detected by in-situ Raman combined with ex-situ characterization methods;
2. It is verified that Bi/Bi2O3 nanosheets are the real active phase in CO2 electroreduction and have high-efficiency formate production capacity;
3. Theoretical calculations indicate that the CO2 electroreduction activity originates from the electron-rich state of surface-exposed Bi supplied by the Bi2O3 substrate.”
“This work explores the structural evolution of the catalyst under cathode conditions and constructs a model to understand the origin of the activity, which provides a reference for the rational design of the CO2 reduction electrocatalyst and the future theoretical research of the activity mechanism,” the scientists estimate.
Journal Reference:
Wang, H., et al. (2022) In-situ structural evolution of Bi2O3 nanoparticle catalysts for CO2 electroreduction. International Journal of Extreme Manufacturing. doi.org/10.1088/2631-7990/ac7a6e.